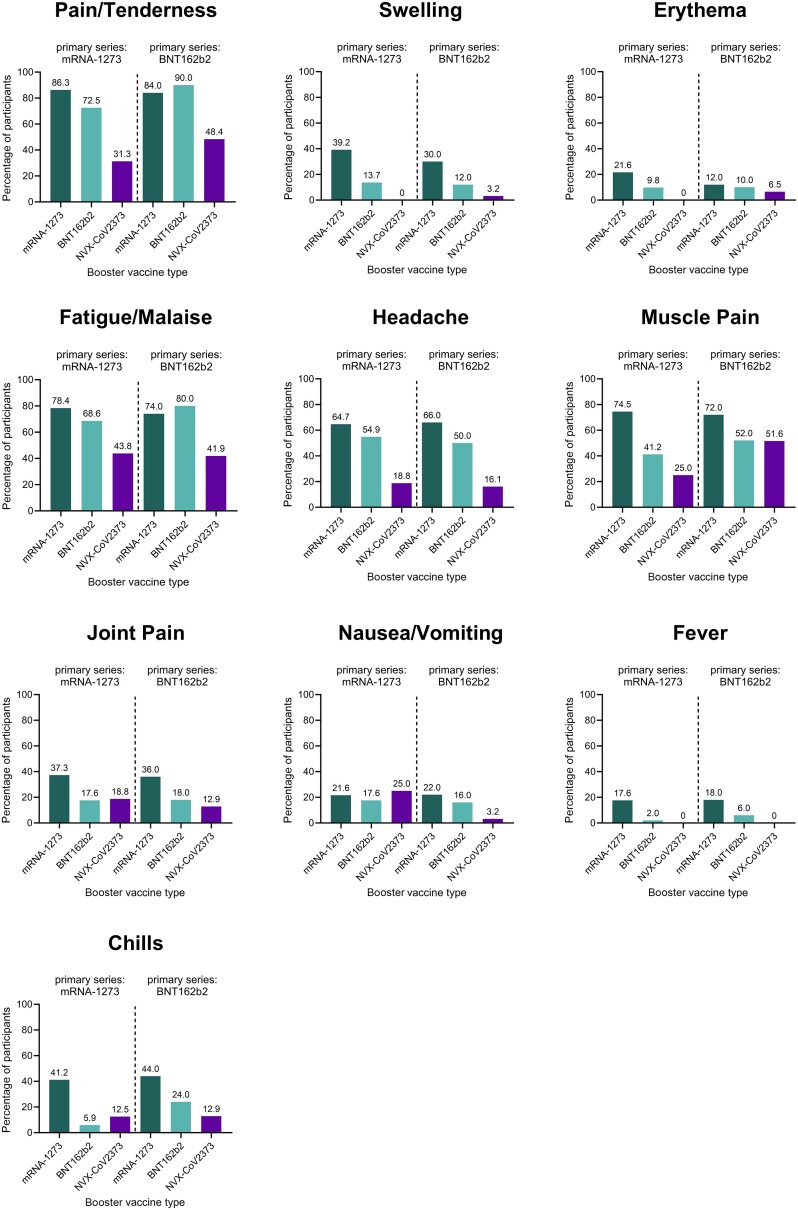
Figure 1.
Summary of postvaccination reactogenic events from the National Institute of Allergy and Infectious Diseases study NCT04889209. All participants received a 2-dose primary series vaccination with mRNA-1273 (Moderna) or BNT162b2 (Pfizer), followed by a single booster dose with mRNA-1273 (dark green), BNT162b2 (teal), or NVX-CoV2373 (Novavax Inc, purple). The percentage of participants experiencing any reactogenic event within 7 days postvaccination is shown for the local symptoms of pain/tenderness, swelling, and erythema, and the systemic events of fatigue/malaise, headache, muscle pain, joint pain, nausea/vomiting, fever, and chills. For 3 homologous doses of mRNA-1273, n = 51; mRNA-1273 (×2)/BNT162b2, n = 51; mRNA-1273 (×2)/NVX-CoV2373, n = 16; 3 homologous doses of BNT162b2, n = 50; BNT162b2(×2)/mRNA-1273, n = 50; BNT162b2 (×2)/NVX-CoV2373, n = 31. Except for pain/tenderness for BNT162b2 (×2)/mRNA-1273, n = 49 (one data point reported as missing). Sources: Atmar et al [26] and Lyke et al [27]. Abbreviations: ×2, 2-dose primary series; BNT, BioNTech; mRNA, messenger RNA; NVX, Novavax.