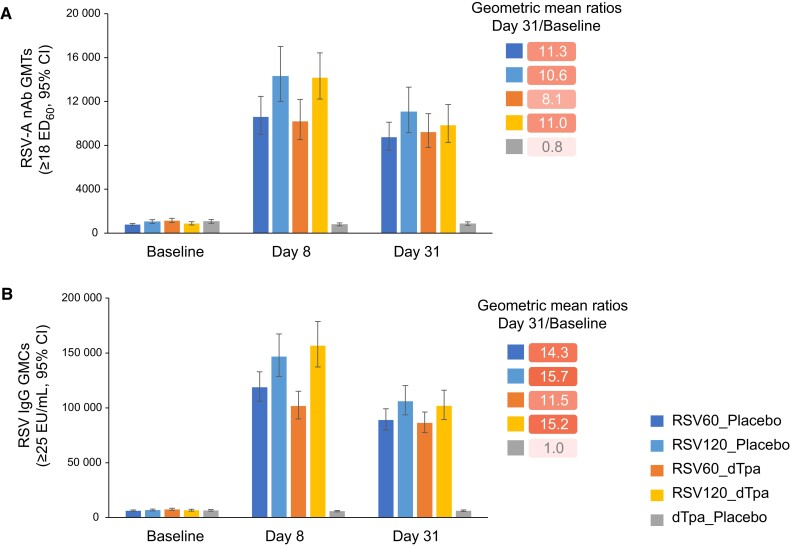
Figure 4.
Humoral immune response in the primary phase to the 2 dose levels (60 and 120 μg) of RSVPreF3 in terms of (A) RSV-A neutralizing antibody geometric mean titers, and (B) RSV immunoglobulin G antibody geometric mean concentrations, at screening, day 8, and day 31 following first-dose vaccination, when given alone and coadministered with dTpa. Abbreviations: CI, confidence interval; dTpa, diphtheria, tetanus, and acellular pertussis; dTpa_placebo, participants who received dTpa and placebo; ED60, serum dilution inducing 60% inhibition in plaque-forming units; EU, enzyme-linked immunosorbent assay unit; GMC, geometric mean concentration; GMT, geometric mean titer; IgG, immunoglobulin G; nAb, neutralizing antibody; RSV, respiratory syncytial virus; RSV60_dTpa, participants who received RSV60 and dTpa; RSV60_placebo, participants who received RSV60 and placebo; RSV120_dTpa, participants who received RSV120 and dTpa; RSV120_placebo, participants who received RSV120 and placebo; RSVPreF3, RSV fusion protein stabilized in the prefusion conformation.