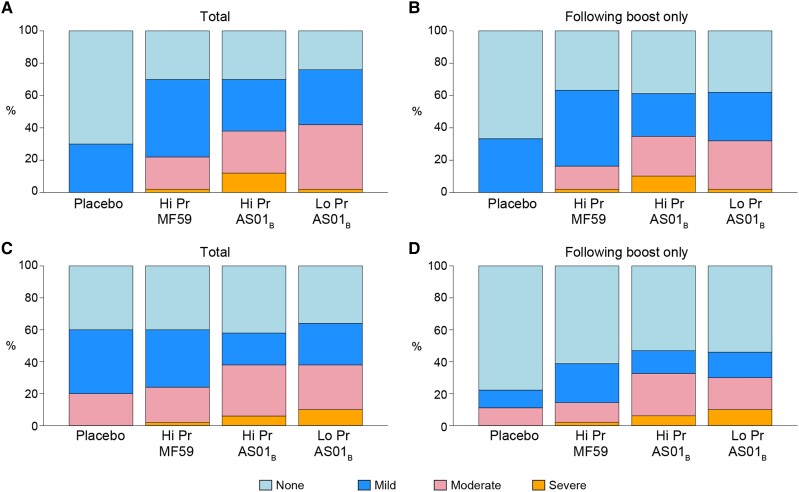
Figure 2.
Maximum local and systemic reactogenicity. Stacked bar charts of maximum (A and B) combined local (pain, tenderness, erythema, induration) and (C and D) combined systemic (malaise/fatigue, myalgia, headache, nausea, vomiting, chills, arthralgia, temperature) reactogenicity. A and C, Reactogenicity over all vaccinations among placebo (n = 10), ALVAC-HIV with 200 µg Env protein + MF59 adjuvant boost (Hi Pr + MF59, n = 50), ALVAC-HIV with 200 µg Env protein + AS01B adjuvant boost (Hi Pr + AS01B, n = 50), and ALVAC-HIV with 40 µg Env protein + AS01B adjuvant boost (Lo Pr + AS01B, n = 50). B and D, Reactogenicity over participants receiving 1 or more boost (third and fourth) vaccinations among placebo (n = 9), Hi Pr + MF59 (n = 49), Hi Pr + AS01B (n = 49), and Lo Pr + AS01B (n = 50). Grade 3 (severe), grade 2 (moderate), grade 1 (mild), none.