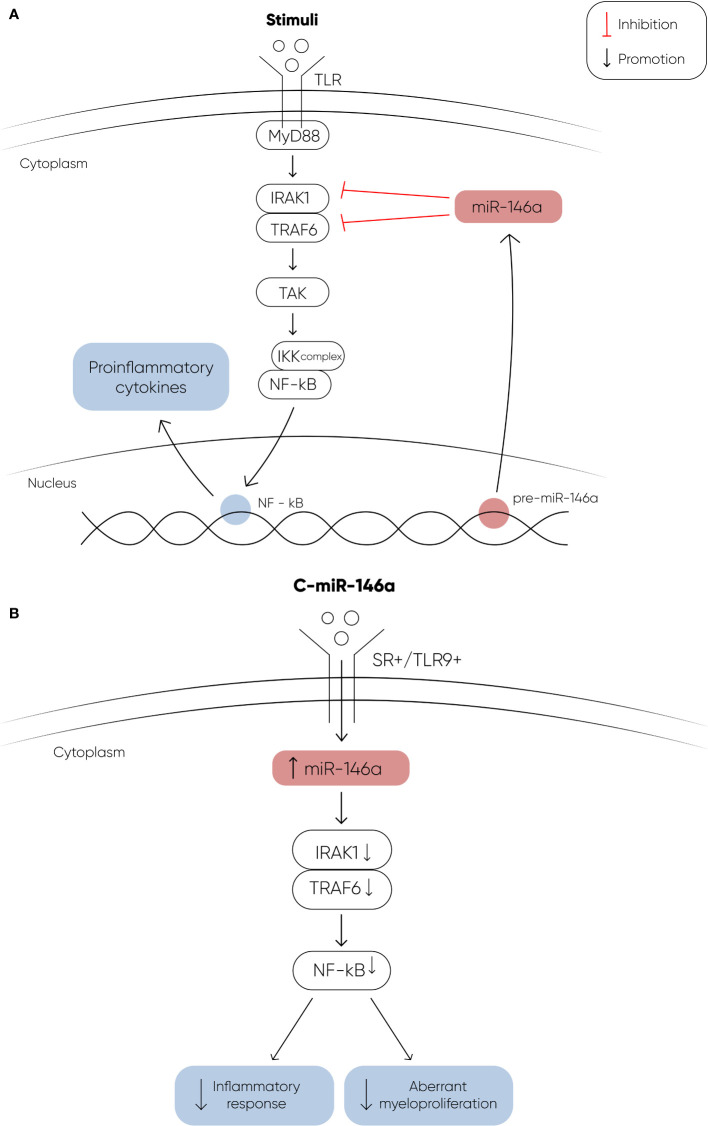
Figure 2.
(A) The figure depicts the signaling cascade initiated by Toll-like receptors (TLRs) upon ligand binding, and the regulatory role of miR-146a in this pathway. Upon binding to their ligands, TLRs undergo a conformational change that recruits the adapter protein MyD88, leading to the activation of IRAK1. Activated IRAK1 then binds to TRAF6, which in turn activates TAK1. TAK1 phosphorylates the IKK complex, resulting in the activation of the transcription factor NF-κB. Activated NF-κB translocates into the nucleus to induce the expression of proinflammatory genes. MiR-146a targets and downregulates IRAK1 and TRAF6, thereby modulating this signaling pathway. In MDS, decreased levels of miR-146a contribute to the activation of NF-κB through IRAK1 and TRAF6, promoting the development of MDS and its progression to AML. (B) The therapeutic potential of a chemically modified miRNA-146a mimic oligonucleotide (C-miR146a) conjugated to a scavenger receptor/Toll-like receptor 9 agonist. This conjugation significantly increases the levels of miR-146a, effectively restoring its function. The restoration of miR-146a levels results in near-complete and durable inhibition of its targets, IRAK1 and TRAF6. This leads to the complete elimination of exacerbated NF-κB activity, thereby preventing exaggerated inflammatory responses and aberrant myeloproliferation (107, 108).