Abstract
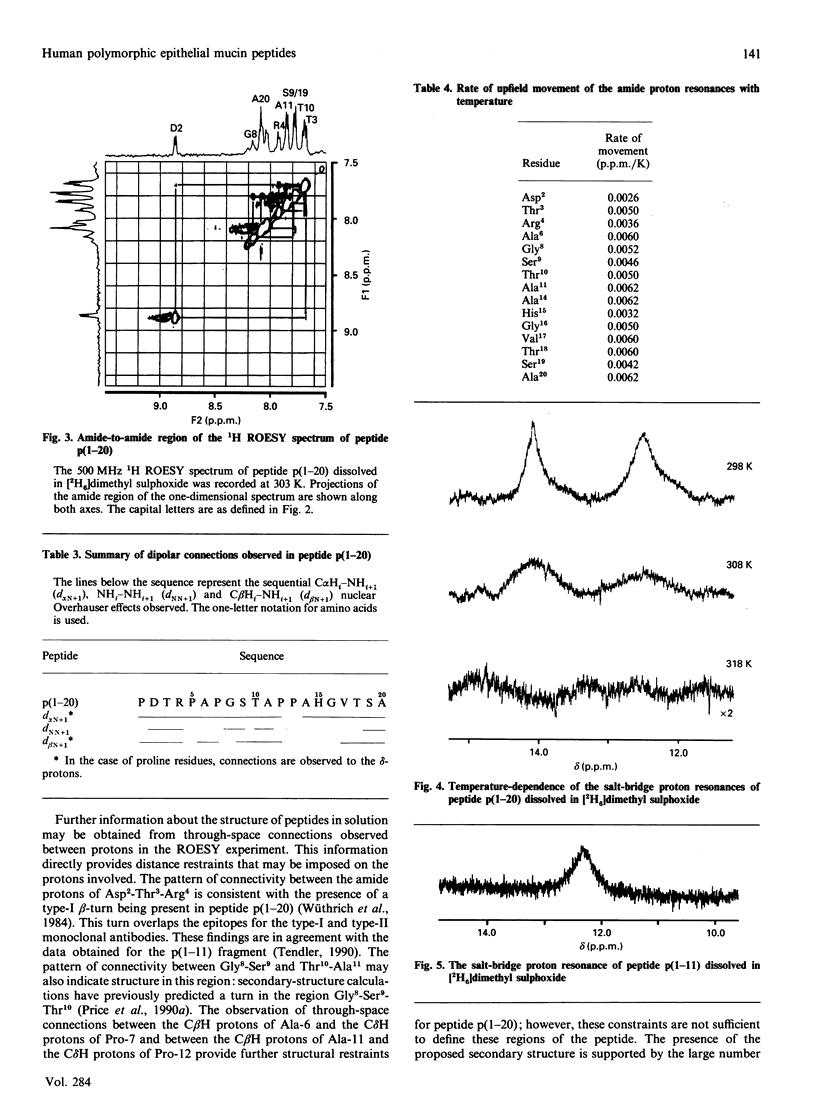
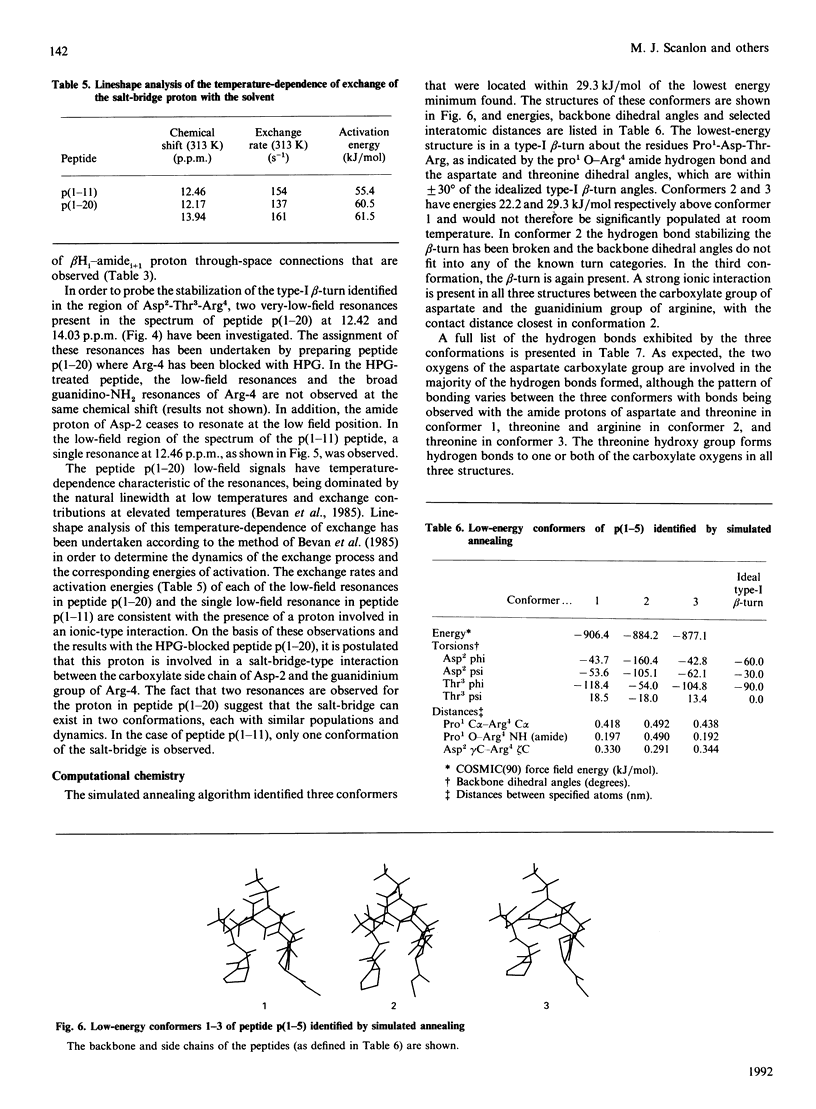
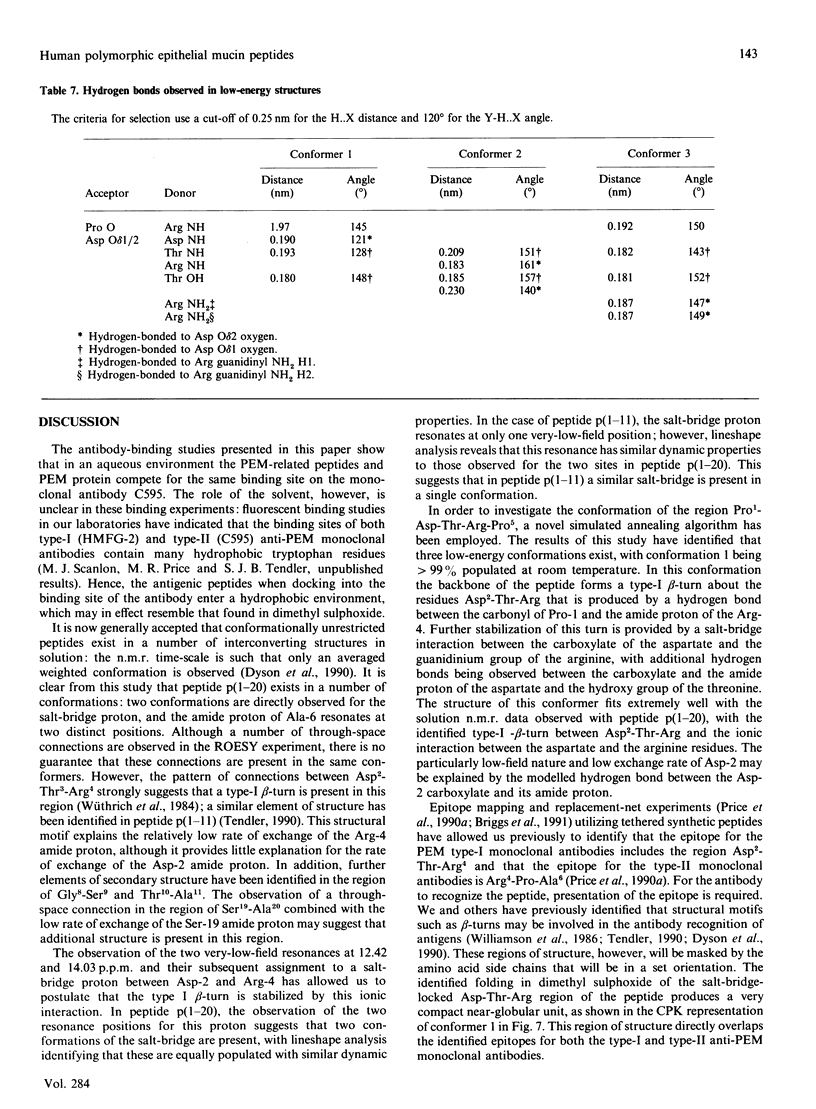
Human polymorphic epithelial mucins (PEM) are complex glycoproteins that are associated with breast and ovarian carcinomas. The PEM core protein consists of variable numbers of a tandem repeat sequence which contains a short antigenic hydrophilic region (Pro1-Asp-Thr-Arg-Pro-Ala-Pro7). High-field n.m.r. studies undertaken on antigenic 20- and 11-amino acid fragments of the PEM core protein in dimethyl sulphoxide have identified a type-I beta-turn to be present in the region Pro1-Asp-Thr-Arg4. This region includes and overlaps the identified type-I (Asp2-Thr-Arg4) and type-II (Arg4-Pro-Ala6) epitopes of anti-PEM monoclonal antibodies. The studies indicate that the beta-turn is stabilized by the presence of a salt-bridge interaction between Asp-2 and Arg-4. In order to probe the conformations accessible to the PEM peptides a computational study was undertaken independently on the peptide Pro-Asp-Thr-Arg-Pro using a modified Metropolis Monte Carlo algorithm. This study identified the n.m.r.-observed salt-bridge type-I beta-turn as the major low-energy conformer. These results suggest that this structural motif may be involved in the immune recognition of PEM.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham R. J., Grant G. H., Haworth I. S., Smith P. E. Charge calculations in molecular mechanics. Part 8. Partial atomic charges from classical calculations. J Comput Aided Mol Des. 1991 Feb;5(1):21–39. doi: 10.1007/BF00173468. [DOI] [PubMed] [Google Scholar]
- Bevan A. W., Roberts G. C., Feeney J., Kuyper L. 1H and 15N NMR studies of protonation and hydrogen-bonding in the binding of trimethoprim to dihydrofolate reductase. Eur Biophys J. 1985;11(4):211–218. doi: 10.1007/BF00261997. [DOI] [PubMed] [Google Scholar]
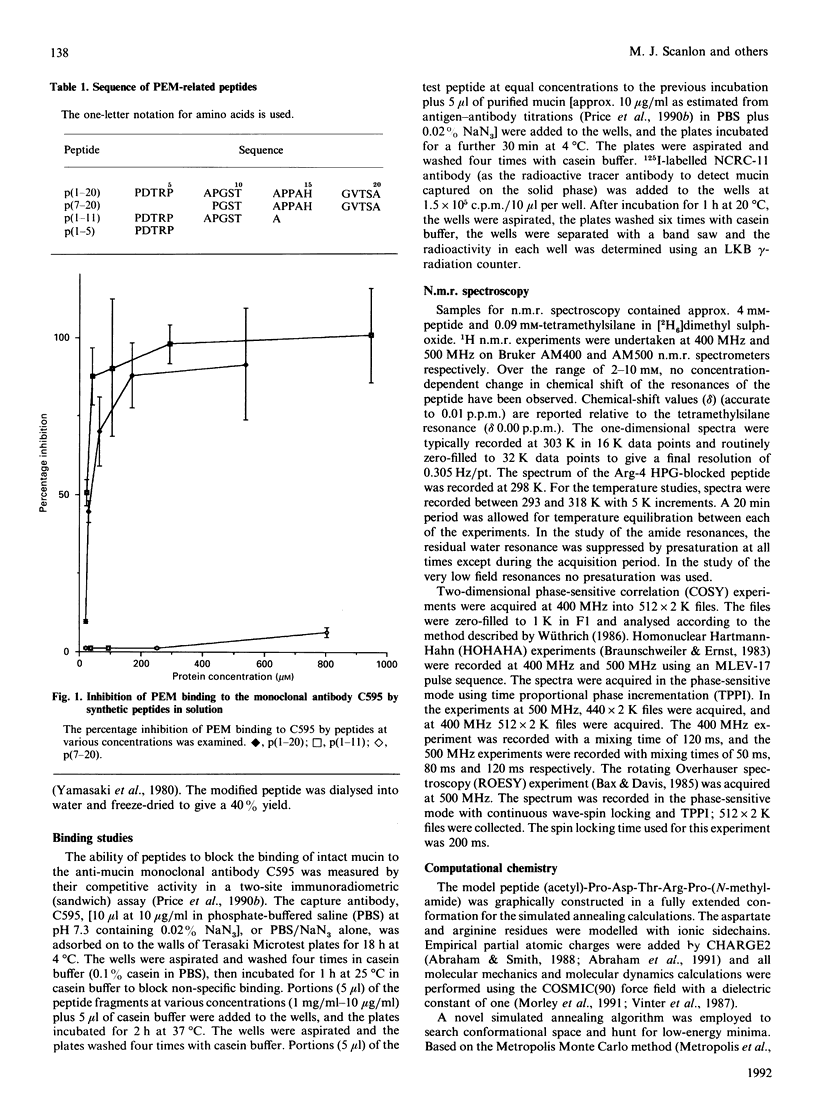
- Briggs S., Price M. R., Tendler S. J. Immune recognition of linear epitopes in peptide fragments of epithelial mucins. Immunology. 1991 Aug;73(4):505–507. [PMC free article] [PubMed] [Google Scholar]
- Dyson H. J., Satterthwait A. C., Lerner R. A., Wright P. E. Conformational preferences of synthetic peptides derived from the immunodominant site of the circumsporozoite protein of Plasmodium falciparum by 1H NMR. Biochemistry. 1990 Aug 28;29(34):7828–7837. doi: 10.1021/bi00486a008. [DOI] [PubMed] [Google Scholar]
- Ellis I. O., Robins R. A., Elston C. W., Blamey R. W., Ferry B., Baldwin R. W. A monoclonal antibody, NCRC-11, raised to human breast carcinoma. 1. Production and immunohistological characterization. Histopathology. 1984 May;8(3):501–516. doi: 10.1111/j.1365-2559.1984.tb02360.x. [DOI] [PubMed] [Google Scholar]
- Gendler S., Taylor-Papadimitriou J., Duhig T., Rothbard J., Burchell J. A highly immunogenic region of a human polymorphic epithelial mucin expressed by carcinomas is made up of tandem repeats. J Biol Chem. 1988 Sep 15;263(26):12820–12823. [PubMed] [Google Scholar]
- Morley S. D., Abraham R. J., Haworth I. S., Jackson D. E., Saunders M. R., Vinter J. G. COSMIC(90): an improved molecular mechanics treatment of hydrocarbons and conjugated systems. J Comput Aided Mol Des. 1991 Oct;5(5):475–504. doi: 10.1007/BF00125666. [DOI] [PubMed] [Google Scholar]
- Price M. R., Hudecz F., O'Sullivan C., Baldwin R. W., Edwards P. M., Tendler S. J. Immunological and structural features of the protein core of human polymorphic epithelial mucin. Mol Immunol. 1990 Aug;27(8):795–802. doi: 10.1016/0161-5890(90)90089-i. [DOI] [PubMed] [Google Scholar]
- Price M. R., Pugh J. A., Hudecz F., Griffiths W., Jacobs E., Symonds I. M., Clarke A. J., Chan W. C., Baldwin R. W. C595--a monoclonal antibody against the protein core of human urinary epithelial mucin commonly expressed in breast carcinomas. Br J Cancer. 1990 May;61(5):681–686. doi: 10.1038/bjc.1990.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swallow D. M., Griffiths B., Bramwell M., Wiseman G., Burchell J. Detection of the urinary 'PUM' polymorphism by the tumour-binding monoclonal antibodies Ca1, Ca2, Ca3, HMFG1, and HMFG2. Dis Markers. 1986 Dec;4(4):247–254. [PubMed] [Google Scholar]
- Taylor-Papadimitriou J., Peterson J. A., Arklie J., Burchell J., Ceriani R. L., Bodmer W. F. Monoclonal antibodies to epithelium-specific components of the human milk fat globule membrane: production and reaction with cells in culture. Int J Cancer. 1981 Jul 15;28(1):17–21. doi: 10.1002/ijc.2910280104. [DOI] [PubMed] [Google Scholar]
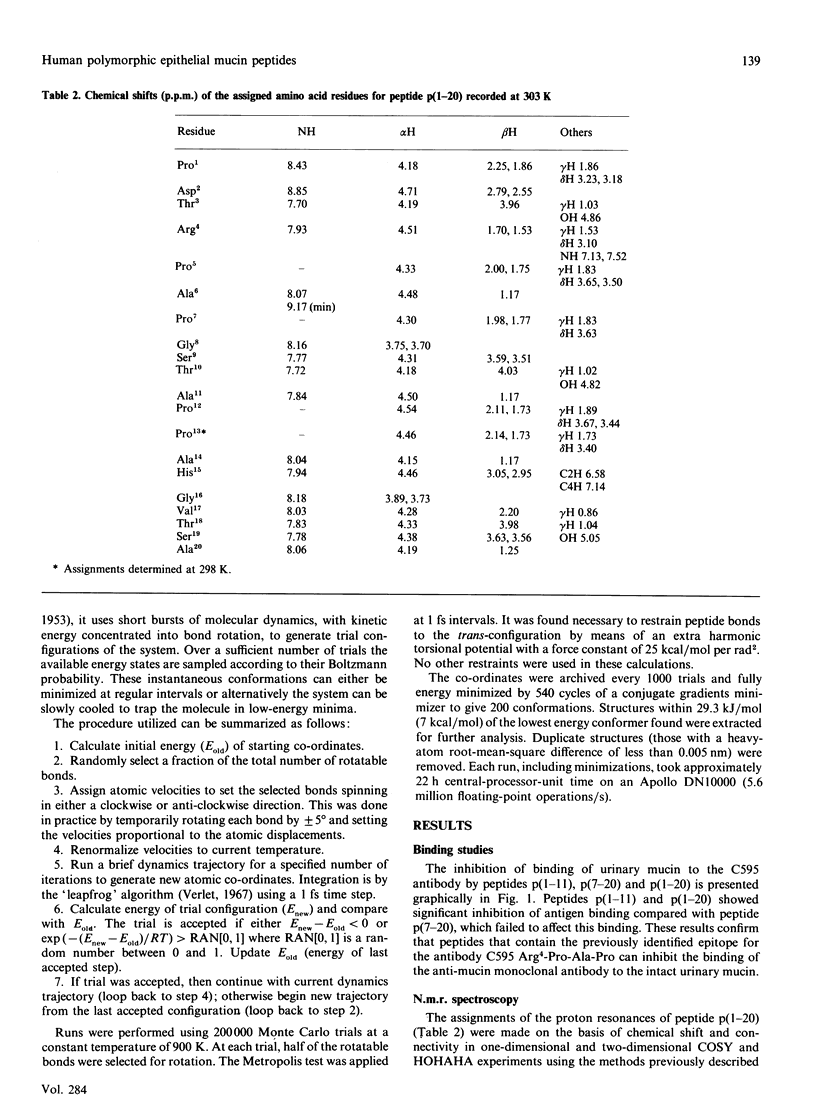
- Tendler S. J. Elements of secondary structure in a human epithelial mucin core peptide fragment. Biochem J. 1990 May 1;267(3):733–737. doi: 10.1042/bj2670733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinter J. G., Davis A., Saunders M. R. Strategic approaches to drug design. I. An integrated software framework for molecular modelling. J Comput Aided Mol Des. 1987 Apr;1(1):31–51. doi: 10.1007/BF01680556. [DOI] [PubMed] [Google Scholar]
- Williamson M. P., Hall M. J., Handa B. K. 1H-NMR assignment and secondary structure of a herpes simplex virus glycoprotein D-1 antigenic domain. Eur J Biochem. 1986 Aug 1;158(3):527–536. doi: 10.1111/j.1432-1033.1986.tb09786.x. [DOI] [PubMed] [Google Scholar]
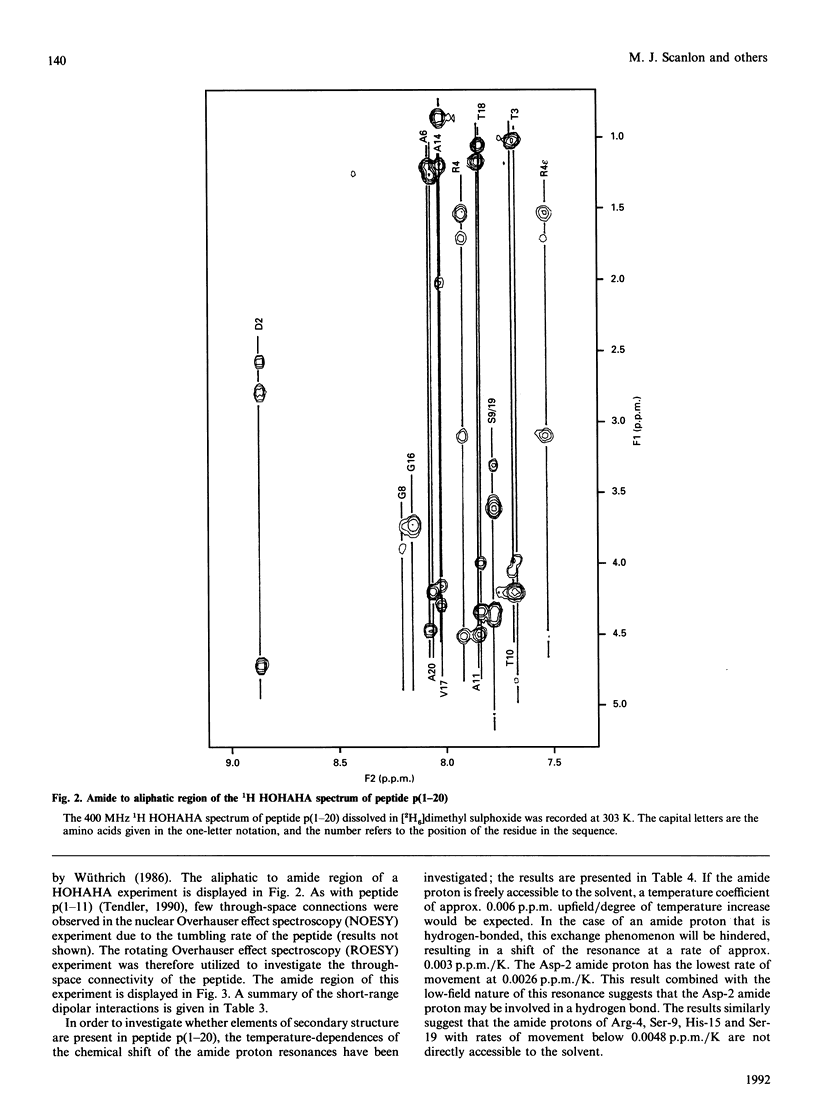
- Wüthrich K., Billeter M., Braun W. Polypeptide secondary structure determination by nuclear magnetic resonance observation of short proton-proton distances. J Mol Biol. 1984 Dec 15;180(3):715–740. doi: 10.1016/0022-2836(84)90034-2. [DOI] [PubMed] [Google Scholar]
- Yamasaki R. B., Vega A., Feeney R. E. Modification of available arginine residues in proteins by p-hydroxyphenylglyoxal. Anal Biochem. 1980 Nov 15;109(1):32–40. doi: 10.1016/0003-2697(80)90006-8. [DOI] [PubMed] [Google Scholar]