Abstract
Intestinal protists in the gut microbiome are increasingly studied, but their basic epidemiology is not well understood. We explored the prevalence, genetic diversity, and potential zoonotic transmission of two protists colonizing the large intestine - Blastocystis sp. and Dientamoeba fragilis - in 37 species of non-human primates (NHPs) and their caregivers in six zoos in the Czech Republic. We analyzed 179 fecal samples (159 from NHPs, 20 from humans) by qPCR. Blastocystis sp. was detected in 54.7% (98/179) of samples, in 24 NHP species and in 57.2% of NHP samples (prevalence ranged between 36 and 80%), and in 35% of human samples (prevalence ranged between 0 and 67%). Using next generation amplicon sequencing, nine Blastocystis subtypes (ST1-ST5, ST7, ST8, and two novel subtypes) were identified. The two new Blastocystis subtypes (named ST47 and ST48) were described using Nanopore sequencing to produce full-length reference sequences of the small subunit ribosomal RNA gene. Some subtypes were shared between NHPs and their caregivers, suggesting potential zoonotic transmission. Mixed subtype colonization was frequently observed, with 52% of sequenced samples containing two or more subtypes. Dientamoeba was found only in NHPs with a prevalence of 6%. This study emphasizes the critical role of molecular diagnostics in epidemiological and transmission studies of these protists and calls for further research to better understand their impact on public health.
Keywords: Blastocystis, Dientamoeba fragilis, Intestinal protists, Non-human primates, Caregivers, Epidemiology, Molecular diagnostics, Nanopore sequencing, Subtypes, Zoonotic transmission
Highlights
-
•
High Blastocystis prevalence: 57.2% in NHPs, 35% in caregivers.
-
•
Discovery of novel Blastocystis subtypes ST47 and ST48 in NHPs.
-
•
Evidence of zoonotic transmission of Blastocystis in zoos.
-
•
Comprehensive molecular methods revealed mixed colonization patterns.
-
•
Dientamoeba fragilis found only in NHPs, absent in human samples.
1. Introduction
The study of intestinal protists, which is becoming increasingly important with the development of research on the gut microbiome, emphasizes the complexity of their role in the host organism. Although the existence of many of these organisms has been known for more than a century [1,2], their function in the gut ecosystem and in epidemiology remains unclear. The protists Blastocystis sp. and Dientamoeba fragilis, both of which colonize the large intestine, have been the subjects of increased research focus in recent years [2,3]. Blastocystis sp., which is found in almost one billion people worldwide, is being intensively studied for its potential positive effect on the gut microbiome as well as to understand what may result in its presence causing symptomatic infection [[4], [5], [6]]. Likewise, D. fragilis remains an enigma in many aspects of its biology and epidemiology. Both protists have been associated with healthy individuals as well as with gastrointestinal problems and diseases such as diarrhea, irritable bowel syndrome or inflammatory bowel disease [5,7,8]. Understanding the underlying causes of these differences in clinical manifestations has led to increased interest in studies of both organisms.
The epidemiology of Blastocystis sp. and D. fragilis is not yet fully understood. A high prevalence of Blastocystis sp., sometimes as high as 100%, has been found in low-income countries, which is attributed to lower hygiene standards, while the prevalence in high-income countries varies between 18% and 24% [[9], [10], [11], [12]]. Transmission of Blastocystis sp. is thought to be primarily via the fecal-oral route via cysts and can be transmitted through contaminated water or food [13,14]. Information on the epidemiology of D. fragilis is much more limited. This protist is cosmopolitan with considerable variation in prevalence, from 0.4% to 91%, depending on geography and diagnostic methods used [2,15]. The mechanism of transmission of D. fragilis between hosts remains unclear [16]. It has been proposed that transmission may occur via Enterobius vermicularis eggs [17,18], and some studies suggest the potential existence of a cyst stage in D. fragilis life cycle [2,19,20]. The recent shift in detection from traditional microscopic methods (concentration methods and smear staining) to more sensitive molecular methods has represented a significant advance in the diagnostics of intestinal protists [21,22]. In the case of Blastocystis sp. several protocols targeting the small subunit ribosomal RNA gene (SSU rRNA) have been developed for both conventional PCR (cPCR) and quantitative PCR (qPCR) and next generation amplicon sequencing (NGS) [12,[22], [23], [24]]. The situation is similar for D. fragilis, although the diagnostic protocols are not as sophisticated as for Blastocystis sp. Even here, it is recommended to favor molecular approaches, especially qPCR [21].
The genetic diversity within Blastocystis sp. is quite broad, in contrast to D. fragilis, where genetic diversity remains completely unexplored. In Blastocystis sp. 42 subtypes have already been distinguished in mammals and birds based on SSU rRNA gene polymorphisms (ST1-ST17, ST21, ST23-ST46), with 16 of those subtypes identified in humans (ST1-ST10, ST12, ST14, ST16, ST23, ST35, and ST41) [24,25], and more subtypes are expected to be discovered in the future [26,27]. Research on D. fragilis is still under development [21,28]. So far, two genotypes have been identified, Genotype 1 and Genotype 2, with most studies reporting a dominance of Genotype 1 [21,29,30]. These two genotypes differ by a sequence difference of about 2% at the SSU rRNA gene [[30], [31], [32]].
In non-human primates (NHPs), 14 Blastocystis subtypes (ST1-ST5, ST7-ST11, ST13, ST15, ST19, ST39) have been reported. ST1, ST2, and ST3 are the most common subtypes in both humans and NHPs [33,34], suggesting the potential for zoonotic transmission. Recent studies in zoological gardens have shown that the likelihood of zoonotic transmission between NHPs and their caretakers is relatively high [[35], [36], [37], [38], [39]]. In contrast, there are far fewer studies on D. fragilis in NHPs [[40], [41], [42]]. The available literature describes the presence of D. fragilis in six NHP species, specifically in wild nature Macaca fascicularis philippensis and Pan troglodytes verus, and captive Macaca spp., Papio spp., Alouatta palliata aequatorialis and Gorilla gorilla gorilla [2,15,40,41].
We aim to improve the epidemiological understanding of the intestinal protists Blastocystis sp. and D. fragilis by providing data on their prevalence and distribution of their subtypes/genotypes in different groups of NHPs and their caregivers in the zoological gardens across the Czech Republic. A major goal of this study is to explore the potential for zoonotic transmission, especially in environments that foster close contact, as is the case in zoos.
2. Materials and methods
2.1. Sample collection
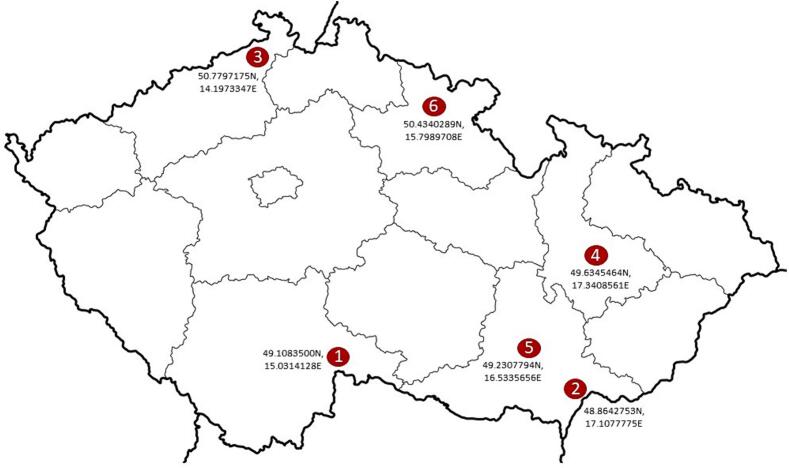
The study was carried out in six zoological gardens (Na Hrádečku Zoo, Hodonín Zoo, Děčín Zoo, Olomouc Zoo, Brno Zoo, and Dvůr Králové Zoo) in the Czech Republic between 2020 and 2022 (for more details about locations and the coordinates see Fig. 1). Fecal samples from non-human primates (NHPs), comprising 37 species (Table 1), were collected from the cage floor during routine maintenance. To avoid cross-contamination, a sterile sampling kit was used, which included a sterile tube, a sterile sampling spatula, and sterile gloves. The number of samples from each zoo was as follows: 11 from Na Hrádečku Zoo, 5 from Hodonín Zoo, 16 from Děčín Zoo, 93 from Olomouc Zoo, 22 from Brno Zoo, and 12 from Dvůr Králové Zoo. The study strictly followed a non-invasive methodology focusing exclusively on the examination of fecal samples. The approval for this research was obtained from all zoological institution to ensure compliance with animal welfare regulations.
Fig. 1.
Map of the Czech Republic showing the locations of the selected zoological gardens. The coordinates for the individual zoos are as follows: (1) Na Hrádečku Zoo (49.1083500 N, 15.0314128E), (2) Hodonín Zoo (48.8642753 N, 17.1077775E), (3) Děčín Zoo (50.7797175 N, 14.1973347E), (4) Olomouc Zoo (49.6345464 N, 17.3408561E), (5) Brno Zoo (49.2307794 N, 16.5335656E), and (6) Dvůr Králové Zoo (50.4340289 N, 15.7989708E).
Table 1.
An overview of the prevalence of Blastocystis sp. and Dientamoeba fragilis in different non-human primate (NHP) species in Czech zoological gardens. The table categorizes each NHP species under its respective family and suborder, along with the total number of samples analyzed. In particular, it shows the percentage prevalence of Blastocystis sp. and D. fragilis for each species, providing insights into the distribution and prevalence of these gut protists in the different groups of NHPs in the zoos studied. [no. – number].
| Host species | Family | No. of samples | Prevalence of Blastocystis sp. | Prevalence of D. fragilis |
|---|---|---|---|---|
| Ateles geoffroyi vellerosus | Atelidae | 4 | 0% (0/4) | 0% (0/4) |
| Callimico goeldii | Callitrichidae | 2 | 100% (2/2) | 0% (0/2) |
| Callithrix jacchus | Callitrichidae | 1 | 0% (0/1) | 0% (0/1) |
| Callithrix penicillata | Callitrichidae | 13 | 0% (0/13) | 7,7% (1/13) |
| Callithrix pygmaea | Callitrichidae | 2 | 0% (0/2) | 0% (0/2) |
| Cercopithecus campbelli | Cercopithecidae | 1 | 0% (0/1) | 0% (0/1) |
| Cercopithecus nictitans | Cercopithecidae | 1 | 100% (1/1) | 100% (1/1) |
| Colobus angolensis | Cercopithecidae | 2 | 100% (2/2) | 0% (0/2) |
| Erythrocebus patas | Cercopithecidae | 6 | 66,7% (4/6) | 0% (0/6) |
| Eulemur albifrons | Lemuridae | 6 | 66,7% (4/6) | 0% (0/6) |
| Eulemur macaco | Lemuridae | 11 | 54,5% (6/11) | 9.1% (1/11) |
| Eulemur rufifrons | Lemuridae | 1 | 100% (1/1) | 0% (0/1) |
| Galago senegalensis | Galagidae | 1 | 0% (0/1) | 0% (0/1) |
| Hylobates lar | Hylobatidae | 5 | 100% (5/5) | 20% (1/5) |
| Chlorocebus sabaeus | Cercopithecidae | 1 | 100% (1/1) | 0% (0/1) |
| Lemur catta | Lemuridae | 20 | 95% (19/20) | 0% (0/20) |
| Leontopithecus rosalia | Callitrichidae | 5 | 0% (0/5) | 20% (1/5) |
| Lophocebus aterrimus | Cercopithecidae | 1 | 100% (1/1) | 0% (0/1) |
| Macaca fuscata | Cercopithecidae | 9 | 100% (9/9) | 0% (0/9) |
| Macaca nigra | Cercopithecidae | 6 | 100% (6/6) | 33.3% (2/6) |
| Macaca radiata | Cercopithecidae | 1 | 100% (1/1) | 0% (0/1) |
| Mandrillus leucophaeus | Cercopithecidae | 3 | 66,7% (2/3) | 0% (0/3) |
| Mico argentatus | Callitrichidae | 2 | 0% (0/2) | 0% (0/2) |
| Miopithecus ogouensis | Cercopithecidae | 1 | 100% (1/1) | 0% (0/1) |
| Nomascus gabriellae | Hylobatidae | 7 | 85,7% (6/7) | 0% (0/7) |
| Otolemur crassicaudatus | Galagidae | 1 | 0% (0/1) | 0% (0/1) |
| Pan troglodytes | Hominidae | 4 | 75% (3/4) | 0% (0/4) |
| Pan troglodytes schweinfurthii | Hominidae | 1 | 100% (1/1) | 0% (0/1) |
| Papio anubis | Cercopithecidae | 1 | 100% (1/1) | 0% (0/1) |
| Pongo pygmaeus | Hominidae | 1 | 100% (1/1) | 0% (0/1) |
| Saguinus imperator | Callitrichidae | 2 | 0% (0/2) | 0% (0/2) |
| Saguinus labiatus | Callitrichidae | 1 | 0% (0/1) | 0% (0/1) |
| Saguinus midas | Callitrichidae | 4 | 0% (0/4) | 0% (0/4) |
| Saimiri sciureus | Cebidae | 18 | 0% (0/18) | 11.1% (2/18) |
| Symphalangus syndactylus | Hylobatidae | 2 | 100% (2/2) | 0% (0/2) |
| Theropithecus gelada | Cercopithecidae | 1 | 100% (1/1) | 0% (0/1) |
| Varecia rubra | Lemuridae | 11 | 100% (11/11) | 9.1% (1/11) |
To assess the zoonotic potential of the studied protists, stool samples were also taken from caregivers. We specifically targeted the caregivers who are responsible for the daily care of the NHPs included in this study, as they have regular daily contact with the NHPs through activities such as cage cleaning, food preparation and other necessary care activities. Caregivers collected their stool samples using the same sterile collection kit as described above. The number of caregivers varied between zoos as follows: 4 from Na Hrádečku Zoo, 2 from Hodonín Zoo, 2 from Děčín Zoo, 4 from Olomouc Zoo, 5 from Brno Zoo and 3 from Dvůr Králové Zoo.
Caregivers' stool samples were obtained on an individual basis, with study participants providing information about the specific NHP species for which they cared. The signed informed consent was obtained with each human sample. The entire process, including sampling conditions and ethical approval, was conducted carefully and in strict accordance with the ethical principles of the World Medical Association based on the Declaration of Helsinki 2013. Caregiver data were anonymized and processed in strict accordance with the applicable laws in the Czech Republic. The study was approved by the Ethics Committee of the Biology Centre of the Czech Academy of Sciences (Proof No. 2/2020).
Both NHP and human samples were immediately sent in ice (4 °C) to the Laboratory of Parasitic Therapy at the Biology Centre of the Czech Academy of Sciences (České Budějovice, Czech Republic), where they were processed.
2.2. DNA extraction and molecular detection
Total DNA extraction from fecal samples was performed using the commercial kit CatchGene Stool DNA Kit (CatchGene, New Taipei City, Taiwan) according to manufacturer's instructions. Resulting DNA aliquots, each containing 200 μl, were stored at -20 °C. To prevent cross-contamination, total DNA was processed under sterile conditions in a DNA/RNA UV cleaner box (UVT-B-AR, Biosan, Riga, Latvia). Samples were handled in small sets, with a maximum of 8 samples processed at the same time and from the same zoo, using filtered tips.
For molecular screening of samples, two different qPCR protocols were used. For the detection of Blastocystis sp. a qPCR that amplifies a 118 bp SSU rRNA gene fragment was used [22], and for D. fragilis a qPCR protocol that amplifies a 97 bp SSU rRNA gene fragment was used [21]. Due to its high sensitivity, qPCR data were used to determine the overall positivity and prevalence of both protists [22]. All samples were tested in triplicate to prevent cross-contamination. If results were ambiguous, the samples were tested until clear results were obtained.
All qPCR analyses were performed using the Light Cycler LC 480 I (Roche, Basel, Switzerland) and under cycling conditions - 95 °C/10 min; 50 × (95 °C / 15 s, 60 °C / 30s, 72 °C / 30s), using the Master Mix HOT FIREPol Probe qPCR Mix Plus Rox (Solis BioDyne, Tartu, Estonia). As positive controls, Blastocystis ST3 and D. fragilis (Genotype 1) DNA from xenic cultures maintained under laboratory conditions were used.
In the case of Blastocystis sp. intensity of colonization was quantified. Briefly, the fecal Blastocystis load was estimated based on a quantification curve established from dilution series of cultured Blastocystis ST3 as previously described [22]. The culture was adjusted to a range of 100–105 cells per 1 qPCR reaction (100–101 – mild fecal protist load; 102–103 – moderate fecal protist load; 104–105 – high fecal protist load). Cell numbers from culture were calculated using a Bürker's chamber and then serially diluted to obtain aliquots of 101, 102, 103, 104 and 105 cells, which were subsequently subjected to DNA extraction.
In negative samples, verification of the absence of PCR inhibition was done for samples negative for both protists. For this purpose, foreign DNA from experimental rat tissue was introduced and a qPCR protocol was applied [22] using commercially available primers and a Taqman probe for the rat gene for beta-2-microglobulin (Thermo Fischer Scientific, Waltham, MA, USA).
2.3. Subtyping of Blastocystis sp.
Blastocystis-positive samples by qPCR were subjected to NGS to determine subtypes according to the method previously described [43]. All samples were tested in duplicate to prevent cross-contamination. If results were ambiguous, the samples were retested until clear results were obtained.However, due to the lower sensitivity of the NGS approach compared to qPCR [22], it could not be used to determine positivity or prevalence for subtyping.
Briefly, amplicons of the Santin region (∼ 500 bp) of the SSU rRNA gene were generated with overhang Illumina primers, purified and sequencing adapters were added with a limited number of PCR cycles with combinatorial indices (Nextera XT Index Kit v2 Set A and D, Illumina, San Diego, CA, USA). Amplicon libraries were purified and balanced on SequalPrep plates (Thermo, Waltham, MA, USA), pooled, supplemented with 20% PhiX control to balance the amplicon signal, and sequenced on a MiSeq instrument using Reagent Kit v2, 2 × 250 bp (Illumina, San Diego, CA, USA). The resulting sequences were downloaded from BaseSpace as demultiplexed fastq files and processed using the program USEARCH10 [44]: Primers were trimmed, reads were filtered for quality, and unique sequences were defined as operational taxonomic units with zero radius, denoized, their abundance was tabulated, off-target amplicons were removed, and subtypes of Blastocystis sp. were identified by clustering with a reference set of representative sequences.
To confirm potential zoonotic transmission of Blastocystis sp. between NHPs and their caregivers, we analyzed intra-subtype variability (i.e., identification of allelle within a given subtype) similarly as previously described [22]. First, we selected samples from both NHPs and caregivers with the same subtype based on NGS results. Then, we obtained partial SSU rRNA gene sequences (∼ 650 bp) using a barcoding protocol [45] followed by Sanger sequencing (Eurofins GATC Biotech, Konstanz, Germany). These sequences were subjected to allele determination on the platform (http://pubmlst.org/blastocystis/).
2.4. Generation of full-length SSU rRNA gene sequences for novel Blastocystis subtypes
A NHP sample (Z21) observed to contain two potentially novel STs of Blastocystis sp. was used to obtain the full-length SSU rRNA gene nucleotide sequences needed to validate these sequences as new subtypes. Full-length SSU rRNA gene sequences were generated using a previously described Nanopore based sequencing strategy [26,46]. This strategy has been demonstrated to produce consensus sequences with accuracies that exceed 99% when compared to Sanger sequencing and MiSeq sequencing [46]. This protocol uses MinION-tailed primers that amplify the full-length SSU rRNA gene sequence of most eukaryotic organisms. The sequencing library was prepared using the Oxford Nanopore Technologies (ONT) SQK-LSK112/114 Ligation Sequencing Kits and EXP-PBC001 PCR Barcoding Kit (ONT) following the manufacturer's protocol for Ligation Sequencing Amplicons—PCR Barcoding (SQK-LSK114 with EXP-PBC001). The final library was loaded onto a MinION Flow Cell (R10.4.1) and sequenced on a MinION Mk1C. Following sequencing, Guppy v6.1.2 was used for base calling, and only reads between 1600 and 2000 nucleotides were retained. Megablast (blast+ v2.13.0) was used to align reads against NCBI's nt database and reads with best hits to Blastocystis sp. references were extracted, dereplicated and clustered using VSEARCH (vsearch v2.15.1) at a 98% identity threshold and checked for chimeras. Clusters were polished with Medaka v1.6.0 and clustered a final time. The full-length nucleotide sequences of the SSU rRNA gene generated in this study were deposited in GenBank under the accession numbers PP847211 and PP847212.
2.5. Phylogenetic and pairwise analysis of full-length reference sequences
A phylogenetic tree was generated including the Blastocystis sp. full-length SSU rRNA gene nucleotide sequences obtained from sample Z21 in this study and reference nucleotide sequences for all currently validated STs obtained from GenBank. Proteromonas lacertae, a Stramenopile closely related to Blastocystis, was used as an outgroup. Sequences were aligned with the Clustal W algorithm, phylogenetic analyses performed using the neighbor-joining (NJ) method, and pairwise distances calculated with the Kimura 2-parameter model [47] using MEGA 11 [48]. All ambiguous positions were removed for each sequence pair (pairwise deletion option). There were a total of 1981 positions in the final dataset. Bootstrapping with 1000 replicates was used to determine support for the clades generated.
2.6. Subtyping of Dientamoeba fragilis
All positive samples from qPCR were analyzed by cPCR to determine subtype using a previously described protocol [21,49].
3. Results
3.1. Prevalence and subtype diversity of Blastocystis sp.
A total of 179 samples from 37 species of NHPs and 20 samples from their caregivers were obtained in six zoological gardens in the Czech Republic, namely Na Hrádečku Zoo, Hodonín Zoo, Děčín Zoo, Olomouc Zoo, Brno Zoo, and Dvůr Králové Zoo (Table 1, Fig. 1).
The overall positivity (human and NHPs) for Blastocystis sp. obtained by qPCR was 54.7% (98/179). The overall prevalence in caregivers and NHPs was 35% (7/20) and 57.2% (91/159), respectively. The prevalence in both humans and NHPs was based on the qPCR data due its high sensitivity as described previously [22]. Detailed results for the individual NHP species/families can be found in Table 1 and Supplementary data 1.
Table 3.
Theoverview of Blastocystis sp. prevalence and subtype diversity in different families of non-human primates (NHP). It includes the number of samples collected per family, the percentage prevalence and the subtypes identified within each family.
| Family | No. of samples | Prevalence | Identified subtypes |
|---|---|---|---|
| Atelidae | 4 | 0% (0/4) | – |
| Callitrichidae | 32 | 6,25% (2/32) | ST2–100% (2/2) |
| Cebidae | 18 | 0% (0/18) | – |
| Cercopithecidae | 34 | 88,2% (30/34) | ST1–10% (3/30) |
| ST3–3.3% (1/30) | |||
| ST8–3.3% (1/30) | |||
| ST1/ST2–3.3% (1/30) | |||
| ST1/ST3–56.7% (17/30) | |||
| ST1/ST2/ST3–20% (6/30) | |||
| Galagidae | 2 | 0% (0/2) | – |
| Hominidaea | 6 | 83,3% (5/6) | ST1–20% (1/5) |
| ST2–20% (1/5) | |||
| ST5–20% (1/5) | |||
| Hylobatidae | 14 | 92,9% (13/14) | ST1–23% (3/13) |
| ST2–7.7% (1/13) | |||
| ST3–7.7% (1/13) | |||
| ST8–15.4% (2/13) | |||
| ST1/ST2–7.7% (1/13) | |||
| ST1/ST8–23% (3/13) | |||
| ST2/ST3–7.7% (1/13) | |||
| ST1/ST2/ST8–7.7% (1/13) | |||
| Lemuridae | 49 | 83,7% (41/49) | ST1–26.9% (11/41) |
| ST2–7.3% (3/41) | |||
| ST4–2.4% (1/41) | |||
| ST5–17% (7/41) | |||
| ST8–2.4% (1/41) | |||
| ST1/ST8–2.4% (1/41) | |||
| ST2/ST8–29.3% (12/41) | |||
| ST1/ST5/ST8–2.4% (1/41) |
without Homo sapiens.
To explore the diversity of Blastocystis subtypes, 98 qPCR-positive samples from both humans (n = 7) and NHPs (n = 91) were analyzed using an NGS approach.
In the NHPs, the most common subtype was ST1 with 58% positivity (49/84), followed by ST2 with 33% (28/84), ST3 with 31% (26/84), ST8 with 27% (23/84), ST5 with 11% (9/84), and ST4 with 1% (1/84), and two unidentified subtypes (see further). Among caregivers, the prevalence of ST3 was 80% (4/5) and of ST1 60% (3/5). The presence of more than one subtype within a sample was common (52%; 46/89) among the Blastocystis-positive samples analyzed by NGS, specifically 52% (44/84) in NHPs and 40% in humans (2/5). In NHPs, mixtures were mainly comprised of two subtypes [ST1 + ST2, ST1 + ST3, ST1 + ST8, ST2 + ST8, ST2 + ST3, and 2 unidentified STs (see further)]. In some cases, three subtypes (ST1 + ST2 + ST3, ST1 + ST2 + ST8) were detected within a sample (see Table 2 and Table 3 for further details). Two cases of mixed subtype colonization with two subtypes were also observed in caregivers (ST1/ST3, ST1/ST3; Table 2).
Table 2.
A detailed summary of qPCR and NGS analysis results for Blastocystis sp. in samples collected from both non-human primates (NHPs) and human caregivers across all Czech zoological gardens. This table provides a detailed understanding of the distribution and diversity of Blastocystis sp. subtypes within and between different host species as well as insights into the intensity of infection indicated by the Ct values.
| Sample ID | Host | Ct value | NGS | Mixed infection | Subtype | Zoo place |
|---|---|---|---|---|---|---|
| Z8/20 | Colobus anglolensis | 21 | + | − | ST1 (100%) | Na Hrádečku Zoo |
| Z9/20 | Lemur catta | 21 | + | − | ST5 (100%) | |
| Z7/20 | Macaca radiata | 24 | + | + | ST1 (94%), ST3 (6%) | |
| Z6/20 | Eulemur rufifrons | 28 | + | − | ST1 (100%) | |
| Z22/20 | Varecia rubra | 22 | + | − | ST4 (100%) | Hodonín Zoo |
| Z21/20 | Pan troglodytes | 24 | + | + | ST47 (88%), ST48 (4%) | |
| Z19/20 | Hylobates lar | 25 | + | + | ST1 (53%), ST2 (47%) | |
| Z18/20 | Chlorocebus sabaeus | 26 | + | + | ST1 (94%), ST3 (6%) | |
| Z17/20 | Human | 32 | − | − | − | |
| Z27/20 | Human | 21 | + | + | ST1 (89%) ST3 (11%) | Děčín Zoo |
| Z26/20 | Lophocebus aterrimus | 22 | + | + | ST1 (51%), ST3 (49%) | |
| Z31/20 | Macaca nigra | 22 | + | + | ST1 (70%), ST3 (30%) | |
| Z33/20 | Macaca nigra | 22 | + | + | ST1 (50%), ST3 (50%) | |
| Z35/20 | Varecia rubra | 22 | + | + | ST1 (90%), ST8 (10%) | |
| Z29/20 | Macaca nigra | 24 | + | + | ST1 (76%), ST3 (24%) | |
| Z30/20 | Macaca nigra | 24 | + | + | ST1 (73%), ST3 (27%) | |
| Z32/20 | Macaca nigra | 25 | + | + | ST1 (62%), ST3 (38%) | |
| Z34/20 | Varecia rubra | 26 | + | + | ST1 (54%), ST5 (1%), ST8 (45%) | |
| Z25/20 | Macaca nigra | 30 | + | + | ST1 (18%), ST3 (82%) | |
| Z36/20 | Varecia rubra | 31 | − | − | − | |
| Z23/20 | Varecia rubra | 32 | − | − | − | |
| Z37/20 | Varecia rubra | 32 | + | − | ST1 (100%) | |
| Z87/20 | Macaca fuscata | 19 | + | + | ST1 (23%), ST2 (52%), ST3 (25%) | Olomouc Zoo |
| Z91/20 | Macaca fuscata | 19 | + | + | ST1 (26%), ST2 (58%), ST3 (16%) | |
| Z47/20 | Macaca fuscata | 20 | + | + | ST1 (17%), ST2 (52%), ST3 (31%) | |
| Z51/20 | Erythrocebus patas | 21 | + | − | ST1 (100%) | |
| Z76/20 | Macaca fuscata | 21 | + | + | ST1 (72%), ST2 (2%), ST3 (27%) | |
| Z78/20 | Macaca fuscata | 21 | + | + | ST1 (32%), ST2 (38%), ST3 (28%) | |
| Z89/20 | Human | 21 | + | + | ST1 (100%) | |
| Z110/20 | Human | 21 | + | − | ST3 (100%) | |
| Z54/20 | Lemur catta | 22 | + | + | ST2 (68%), ST8 (32%) | |
| Z58/20 | Nomascus gabriellae | 22 | + | + | ST1 (61%), ST2 (19%), ST8 (21%) | |
| Z44/20 | Eulemur macaco | 23 | + | − | ST1 (100%) | |
| Z93/20 | Macaca fuscata | 23 | + | + | ST1 (52%), ST3 (47%) | |
| Z96/20 | Symphalangus syndactylus | 23 | + | + | ST2 (100%) | |
| Z42/20 | Symphalangus syndactylus | 24 | + | + | ST2 (97%), ST3 (3%) | |
| Z49/20 | Hylobates lar | 24 | + | − | ST1 (100%) | |
| Z59/20 | Eulemur macaco | 24 | + | − | ST1 (100%) | |
| Z65/20 | Varecia + Eulemur | 24 | + | + | ST2 (92%), ST8 (8%) | |
| Z68/20 | Lemur catta | 24 | + | + | ST2 (58%), ST8 (42%) | |
| Z74/20 | Eulemur macaco | 24 | + | − | ST1 (100%) | |
| Z100/20 | Erythrocebus patas | 24 | + | + | ST1 (81%), ST3 (19%) | |
| Z101/20 | Lemur catta | 24 | + | + | ST2 (56%), ST8 (44%) | |
| Z41/20 | Lemur cattta | 25 | + | + | ST2 (90%), ST8 (9%) | |
| Z43/20 | Macaca fuscata | 25 | + | + | ST1 (80%), ST3 (20%) | |
| Z53/20 | Lemur catta | 25 | + | + | ST2 (88%), ST8 (12%) | |
| Z55/20 | Erythrocebus patas | 25 | + | − | ST3 (100%) | |
| Z63/20 | Eulemur albifrons | 25 | + | − | ST1 (100%) | |
| Z118/20 | Lemur catta | 25 | + | + | ST2 (53%), ST8 (47%) | |
| Z119/20 | Eulemur albifrons | 25 | + | − | ST1 (100%) | |
| Z135/20 | Nomascus gabriellae | 25 | + | − | ST8 (100%) | |
| Z136/20 | Lemur catta | 25 | + | + | ST2 (70%), ST8 (30%) | |
| Z64/20 | Varecia + Eulemur | 26 | + | + | ST2 (94%), ST8 (6%) | |
| Z66/20 | Hylobates lar | 26 | + | − | ST1 (100%) | |
| Z79/20 | Macaca fuscata | 26 | + | + | ST1 (83%), ST3 (17%) | |
| Z88/20 | Lemur catta | 26 | + | + | ST2 (100%) | |
| Z95/20 | Eulemur macaco | 26 | + | + | ST1 (100%) | |
| Z123/20 | Lemur catta | 26 | + | + | ST2 (37%), ST8 (63%) | |
| Z50/20 | Varecia + Eulemur | 27 | + | − | ST2 (100%) | |
| Z75/20 | Nomascus gabriellae | 27 | + | − | ST8 (100%) | |
| Z98/20 | Lemur catta | 27 | + | + | ST2 (65%), ST8 (35%) | |
| Z115/20 | Nomascus gabriellae | 27 | + | + | ST1 (97%), ST8 (3%) | |
| Z116/20 | Erythrocebus patas | 27 | + | + | ST1 (82%), ST3 (17%) | |
| Z60/20 | Hylobates lar | 28 | + | − | ST1 (100%) | |
| Z71/20 | Callimico goeldii | 28 | + | − | ST2 (100%) | |
| Z97/20 | Lemur catta | 29 | − | − | − | |
| Z99/20 | Lemur catta | 29 | + | + | ST2 (99%), ST8 (1%) | |
| Z45/20 | Nomascus gabriellae | 30 | + | + | ST1 (94%), ST8 (5%) | |
| Z92/20 | Lemur catta | 30 | + | + | ST2 (100%) | |
| Z46/20 | Macaca fuscata | 31 | + | + | ST1 (9%), ST2 (26%), ST3 (65%) | |
| Z69/20 | Nomascus gabriellae | 31 | + | + | ST1 (90%), ST8 (10%) | |
| Z52/20 | Eulemur albifrons | 32 | − | − | − | |
| Z62/20 | Eulemur macaco | 32 | + | − | ST1 (100%) | |
| Z109/20 | Callimico goeldii | 32 | + | − | ST2 (100%) | |
| Z128/20 | Hylobates lar | 32 | + | − | ST3 (100%) | |
| Z131/20 | Eulemur albifrons | 32 | + | − | SA2:F55T1 (100%) | |
| Z152/20 | Eulemur macaco | 19 | + | − | ST5 (100%) | Brno Zoo |
| Z150/20 | Lemur catta | 20 | + | − | ST5 (100%) | |
| Z146/20 | Human | 21 | + | − | ST7 (100%) | |
| Z143/20 | Theropithecus gelada | 24 | + | + | ST1 (65%), ST2 (35%) | |
| Z147/20 | Lemur catta | 24 | + | − | ST5 (100%) | |
| Z148/20 | Lemur catta | 24 | + | − | ST5 (100%) | |
| Z149/20 | Lemur catta | 24 | + | − | ST5 (100%) | |
| Z151/20 | Lemur catta | 24 | + | − | ST5 (100%) | |
| Z138/20 | Pan troglodytes | 32 | − | − | − | |
| Z139/20 | Pan troglodytes | 32 | + | − | ST2 (100%) | |
| Z154/20 | Papio anubis | 32 | − | − | − | |
| Z158/20 | Varecia rubra | 32 | + | − | ST1 (100%) | |
| Z159/20 | Varecia rubra | 32 | + | − | ST8 (100%) | |
| Z178/20 | Human | 16 | + | + | ST1 (27%) ST3 (73%) | Dvůr Králové Zoo |
| Z171/20 | Mandrillus leucophaeus | 19 | + | + | ST1 (14%), ST3 (86%) | |
| Z172/20 | Mandrillus leucophaeus | 23 | + | + | ST1 (47%), ST3 (52%) | |
| Z165/20 | Miopithecus ogouensis | 24 | + | − | ST1 (100%) | |
| Z167/20 | Cercopithecus nictitans | 24 | + | + | ST1 (65%), ST3 (34%) | |
| Z169/20 | Colobus angolensis | 27 | + | − | ST8 (100%) | |
| Z177/20 | Human | 27 | + | − | ST3 (100%) | |
| Z175/20 | Pan troglodytes schweinfurthii | 28 | + | − | ST5 (100%) | |
| Z176/20 | Pongo pygmaeus | 29 | + | − | ST1 (100%) |
3.2. Designation of Blastocystis sp. novel subtypes ST47 and ST48
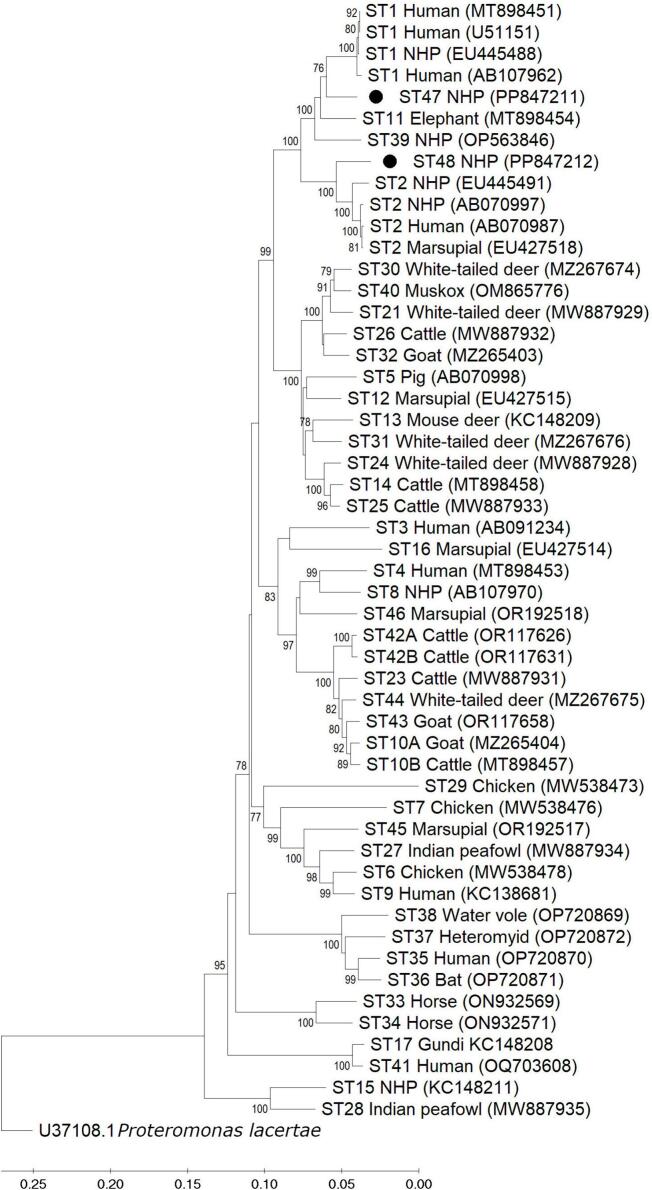
A sample from a chimpanzee from the Hodonín Zoo (Z21) contained two sequences that appeared to represent novel subtypes as they differed from the sequence of any known ST by 6%. To confirm the identification of these sequences as new STs of Blastocystis sp., Nanopore sequencing was used to obtain the full-length sequences of the SSU rRNA gene. Phylogenetic analysis showed that the two potentially novel ST sequences branched with the ST1/ST2/ST11/ST39 clade (Fig. 2). One of the sequences (named ST47) forms a clade with ST1/ST11/ST39 and within this clade ST11, ST39, and novel ST47 formed separate branches. The second novel sequence (named ST48) forms a clade with ST2 but forms a separate and well supported branch within this clade (100%). Pairwise distance analysis of the two new sequences along with full-length reference sequences of all other accepted STs demonstrated a shared sequence identify of 96% for both novel STs (not shown). The most similar STs were ST1/ST2/ST11/ST39, and specific similarity data is shown in Table 4. These data support the designation of two new subtypes named ST47 and ST48.
Fig. 2.
Phylogenetic relationships among Blastocystis full-length SSU rRNA gene nucleotide sequences generated in the present study (represented with filled circles), representative reference sequences of the accepted subtypes (Table 1), and Proteromonas lacertae used as outgroup taxon to artificially root the tree. Analysis was inferred using the neighbor-joining method with genetic distances calculated using the Kimura 2-parameter model. This analysis involved 52 nucleotide sequences, and there were a total of 1981 positions in the final dataset. Bootstrap values lower than 75% are not displayed.
Table 4.
Pairwise distances between Blastocystis sp. full-length SSU rRNA gene sequences for subtypes ST1, ST2, ST11, ST39, ST47, and ST48. It shows the average number of base substitutions per site conducted using the Kimura 2-parameter model.
| ST1 | ST2 | ST11 | ST39 | ST47 | ST48 | |
|---|---|---|---|---|---|---|
| ST1 | ||||||
| ST2 | 0.07 | |||||
| ST11 | 0.05 | 0.08 | ||||
| ST39 | 0.05 | 0.08 | 0.06 | |||
| ST47 | 0.04 | 0.08 | 0.04 | 0.05 | ||
| ST48 | 0.08 | 0.04 | 0.08 | 0.09 | 0.09 |
The first 600 bp of the SSU rRNA gene, sometimes called the barcoding region, is frequently used for Blastocystis subtyping. However, this region has been shown to be too conserved to accurately distinguish between all Blastocystis STs [26]. A BLAST analysis of the first 600 bp of ST47 found that this region of the sequence matches with several sequences previously reported as ST1. ST47 has 99.8% similarity with MZ182327, previously reported as ST1, allele 8 in a wild chimpanzee (Pan troglodytes verus) from Senegal; 99.8% with MZ496541 reported as ST1, allele 7 + 8 in a captive chimpanzee from Sierra Leone; 99.8% with MZ496540 reported as ST1, allele 8 in a captive chimpanzee from Sierra Leone; 99.8% with HQ286905 reported as ST1 in a chimpanzee from Tanzania; 99.5% with MN526862 and MN526861 reported as ST1 in a western lowland gorillas from a UK zoo; 98.8% with JQ974934 reported as ST1 in a chimpanzee from Uganda. Thus, ST47 has likely been observed in NHPs from several countries. When the barcoding region of ST47 is compared to full-length reference sequences of ST1 the sequence similarity is <98% across this region. BLAST analysis of the barcoding region of ST48 demonstrated that this ST shared ≤98.3% identity with any sequences in the database.
3.3. Blastocystis sp. intensity of infection in NHPs and caregivers
To assess the intensity of Blastocystis sp. infection, referred to as “fecal protist load” [22], in the samples from NHPs and caregivers, we used a qPCR quantification curve based on a dilution series of cells from culture. Detailed information on the correlation between cell counts and Ct values as well as information on the subtypes identified by NGS, including mixed colonizations, are displayed in Table 2 and Table 5.
Table 5.
Evaluation of fecal load of Blastocystis sp. in human and NHP samples based on the established quantification curve (set in the range of 100 to 105 per qPCR reaction).
| Fecal protist loada | No. of positive samples / No. samples examined | Ct value range |
|---|---|---|
| 101–102 | 22/179 | 29–32 |
| 103–104 | 68/179 | 21–28 |
| 105–106 | 8/179 | 16–20 |
Number of cells per 1 qPCR reaction.
Our analysis revealed that most samples (68/98) had a mean Ct value between 21 and 28, corresponding to an estimated fecal protist load between 103 and 104. A smaller proportion of samples (22/179) exhibited a lower intensity of infection, with Ct values between 29 and 32, indicating a protist load in the range of 101 to 102. Only eight samples were identified as heavily colonized with Blastocystis sp., characterized by Ct values between 16 and 20, with estimated fecal protist load of 105 to 106 (Table 4).
3.4. Overall occurrence of Dientamoeba fragilis
The overall detection rate of D. fragilis was low at 5.6% (10/179). Interestingly, this protist was not detected in any of the caregiver samples (0/20). However, a prevalence of 6.3% (10/159) was found in NHPs (see Table 1 for details). Detailed results for the individual NHP species and families can be found in Supplementary data 1. Our analysis revealed that the samples had a mean Ct value between 37 and 43.
3.5. Occurrence and genetic diversity of intestinal protists in individual Zoos
3.5.1. Na Hrádečku Zoo
Of the 15 samples collected, 11 were from NHPs and four from caregivers (see Table 1). The overall rate of Blastocystis sp. positivity was 26.7% (4/15), with NHPs showing the prevalence of 36.4% (4/11) (Table 2). None of the caregiver samples tested positive for Blastocystis (0/4). Three subtypes were identified in the NHPs (ST1, ST3, ST5), with one NHP sample (Macaca radiata) showing mixed colonization with two subtypes (ST1/ST3) (Table 2). Dientamoeba fragilis was not detected.
3.5.2. Hodonín Zoo
Seven samples were collected here, five from NHPs and two from caregivers (Table 1). The overall positivity of Blastocystis sp. was 71.4% (5/7), with 80% in NHPs (4/5) and 50% in caregivers (1/2) (Table 2). ST47 and ST48 were found in one chimpanzee and was the only mixed subtype colonization was observed. Dientamoeba fragilis was not detected.
3.5.3. Děčín Zoo
A total of 18 samples were obtained including 16 from NHPs and two from caregivers (Table 1). The overall positivity for Blastocystis sp. was 72.2% (13/18). Among NHPs, the prevalence was 75% (12/16), while among caregivers it was 50% (1/2) (Table 2). We found four subtypes in NHPs (ST1, ST3, ST5, ST8) and two subtypes in caregivers (ST1, ST3). Using NGS, we identified ten samples with mixed subtype colonization, of which one was from the caregiver and nine samples were from the NHP (ST1/ST3 – Macaca nigra, Lophocebus aterrimus; ST1/ST5/ST8 – Varecia rubra; ST1/ST8 – Varecia rubra) (Table 2). The overall prevalence of D. fragilis in NHPs was 16.6% (3/18), while all caregivers were negative.
3.5.4. Olomouc Zoo
This zoo provided the highest number of samples with a total of 97, including 93 samples from NHPs and four from caregivers. The overall positivity for Blastocystis sp. was 55.7% (54/97) (Table 1 and Table 3). Among NHPs, the prevalence was 56% (52/93), and among caregivers 50% (2/4). We found four subtypes in NHPs (ST1, ST2, ST3, ST8) and two subtypes in caregivers (ST1, ST3). The mixed subtype colonization was found in 28 samples originated only from NHPs (Table 2). Dientamoeba fragilis was detected only in samples from NHPs with a prevalence of 5.4% (5/93) (Table 1: and Suplementary data 1).
3.5.5. Brno Zoo
This zoo contributed with a total number of 27 samples, 22 from NHPs and five from caregivers. The overall positivity for Blastocystis sp. was 48.1% (13/27). Among NHPs, the prevalence was 54.5% (12/22) and among caregivers 20% (1/5). We found four subtypes in NHPs (ST1, ST2, ST5, ST8) and ST7 in a caregiver (Table 2). Only one mixed subtype colonization was detected in a sample from a NHP (ST1/ST2 – Theropithecus gelada). Dientamoeba fragilis was detected exclusively in NHP samples, with a prevalence of 3.7% (1/22).
3.5.6. Dvůr Králové Zoo
From this zoo we received a total of 15 samples – 12 from NHPs and three from caregivers. The overall positivity for Blastocystis sp. was 60% (9/15) (Table 2). NHPs showed a prevalence of 58.3% (7/12), while caregivers had a prevalence of 66.7% (2/3). We found four subtypes in NHPs (ST1, ST3, ST5, ST8) and two subtypes in caregivers (ST1, ST3). Mixed subtype colonization was found in one caregiver (ST1/ST3) and three NHPs (ST1/ST3 – Cercopithecus nictitans, Mandrillus leucophaeus). Interestingly, this caregiver had the same subtype combination as the three NHPs, which could indicate possible zoonotic transmission. The positive samples for D. fragilis were exclusively from NHPs with a prevalence of 8.3% (1/12).
3.6. Zoonotic potential
As D. fragilis was not detected in any of the caregivers, our analysis focused primarily on the zoonotic transmission potential of Blastocystis sp. which was found in 35% of the caregivers by qPCR (7/20). Identical NGS sequences observed in both NHPs, and their caregivers were considered evidence of potential zoonotic transmission. Shared Blastocystis sp. colonization, with the same subtypes present in NHPs and caregivers, was observed in Děčín Zoo, Olomouc Zoo and Dvůr Králové Zoo. Shared sequence identity between NHPs and caregivers was further confirmed using the sequences obtained by the barcoding protocol and Sanger sequencing as previously described [22].
In the Děčín Zoo, one caregiver and seven NHPs under his/her care showed mixed colonization with Blastocystis sp. subtypes ST1 and ST3 (Table 2). In the Dvůr Králové Zoo, identical Blastocystis sp. subtypes were found in two caregivers and the NHPs under their care (Table 2). Unfortunately, we could not distinguish the variability within the subtypes using the barcoding protocol due to mixed subtype colonization in all these samples. However, at Olomouc Zoo, we detected two cases of potential zoonotic transmission between caregivers and NHPs involving subtypes ST1 and ST3 based on the NGS results (Table 2). We then obtained sequences using the barcoding protocol and Sanger sequencing. By comparing the sequences with Unipro UGENE software, we identified identical ST1 and ST3 subtype sequences and confirmed the presence of the same alleles (ST1 - allele 1; ST3 - allele 34) in both the caregivers and NHPs. Overall, these results indicate a high probability of zoonotic transmission of Blastocystis sp. between NHPs and caregivers.
4. Discussion
The discussion on the zoonotic transmission of intestinal protists between humans and non-human primates (NHPs), which is an essential part of the One-Health concept, has aroused great interest. This is particularly evident in several studies that emphasize the likelihood of such transmission between NHPs and their caregivers in zoological gardens [2,37,38,50,51]. Köster et al. [39] demonstrated the potential transmission of Blastocystis sp. between NHPs and caregivers in European zoos, despite strict hygiene and sanitation measures [37]. The previous studies of Lhotská et al. [12] and Jirků et al. [21] have shown a significant correlation between direct contact with animals and the presence of these protists in individuals without gastrointestinal symptoms. Our study extends this research by investigating not only the prevalence, but also genetic diversity (including mixed colonization) and potential zoonotic transmission of two commensal protists inhabiting the host hindgut, specifically Blastocystis sp. and Dientamoeba fragilis, in 37 NHP species and their caregivers in Czech zoos. To obtain high quality results we employed a combination of several molecular methods including state-of-the-art metagenomic approaches.
From 2020 to 2022, we collected 179 samples from six zoological gardens in the Czech Republic as part of our study, 159 from NHPs and 20 from caregivers. Using qPCR, we detected Blastocystis sp. in 35.0% of human samples and in 57.2% of samples from NHPs. These results are in close agreement with the findings of Cian et al. [51], who also used qPCR and found a prevalence of 60.3% in NHPs in French zoos. While conventional PCR (cPCR) has a lower sensitivity compared to qPCR [22], similar prevalence rates of 59–63% have been reported in some studies that used cPCR [36,38], in contrast to others reporting lower rates (15.7–37%) [39,[52], [53], [54], [55]]. It is noteworthy that the 35% prevalence of Blastocystis sp. in caregivers is higher than in other European zoo studies, where it ranged between 17.5 and 25.7% [[37], [38], [39]].
In our study, the prevalence of Blastocystis sp. varied between zoos, with the lowest prevalence being 26.6% in the Na Hrádečku Zoo and the highest prevalence being 72.2% in Děčín. Overall, we identified Blastocystis sp. in 37 primate species, with the highest prevalence in the Cercopithecidae family, particularly in Macaca species such as M. fuscata. This high prevalence might be due to the social behavior and close physical interactions within macaque groups. Remarkably, 100% prevalence was found in several Macaca species in three zoos. This is consistent with Zanzani et al. [56], who reported a high prevalence in M. fascicularis, suggesting that these species are potential natural reservoirs for Blastocystis sp. Our results add to the existing knowledge on Blastocystis sp. in NHPs by detecting Blastocystis sp. in previously unreported species such as Miopithecus ogouensis, Theropithecus gelada and Callimico goeldii [34,39,51].
In contrast to Blastocystis sp., the situation with D. fragilis was different in our study. Its presence was confirmed exclusively in eight NHP species in three zoos with a prevalence of 5.6%, based on qPCR, and no human sample was positive. Our results extend the existing knowledge on the distribution of D. fragilis as we describe its occurrence in seven previously unrecorded NHP's genera (Varecia rubra, Cercopithecus nictitans, Saimiri sciureus, Callithrix penicillata, Eulemur macaco, Leontopithecus rosalia, Hylobates lar) and furthermore in an additional species from the genus Macaca, specifically in M. nigra.
Historically, D. fragilis was first identified in baboons of the genus Papio [2]. Another important study in this area was the work of Stark et al. [42], which focused on the prevalence of D. fragilis in various species of NHPs at the Taronga Zoo, including Gorilla gorilla gorilla and Pan troglodytes, and found a 30% prevalence of D. fragilis exclusively in G. gorilla gorilla using cPCR. A key study by Menu et al. [41] used qPCR to investigate the presence of D. fragilis in NHPs in two natural reserves (Dindifelo Community Natural Reserve in Senegal and Lesio Louna Lefini Gorilla in the Republic of Congo) and in the Beauval Zoo in France. There, D. fragilis was found in the wild Pan troglodytes with a prevalence of 25%, while all G. gorilla gorilla in the zoo were negative. There is further records of D. fragilis in NHP, but these are based on studies using only traditional coprological methods for diagnosis [40,42,57]. While the first two studies identified D. fragilis in G. gorilla gorilla, Helenbrook et al. [40] reported its occurrence in Alouatta palliata aequatorialis in Ecuador with a prevalence of 3%. These available data indicate that D. fragilis occurs in NHPs both in captivity and in the wild. However, due to differences in the sensitivity of the diagnostic methods used, it is difficult to get an accurate idea of the real prevalence of this intestinal protist in NHPs.
Surprisingly, we did not detect D. fragilis in any of the caregivers across the zoos. This could be due to the low number of samples and the previously described low prevalence (24%) in the Czech human population [21]. Therefore, we could not assess potential zoonotic transmission.
Genetic diversity, which could provide valuable information on possible zoonotic transmission between NHPs and caregivers, has only been studied here in detail in the protist Blastocystis sp. The reason for this is the detailed methodology used to determine its subtypes, including differentiation at the level of intra-subtype variability [1,26]. Contrary to this, we could not satisfactorily examine genetic diversity in D. fragilis using current methods. In addition, the D. fragilis-positive NHPs samples generally showed low colonization intensity as determined by Ct values from qPCR, and in such cases, sequencing could not be performed using standard procedures as described in our previous study Jirků et al., [21].
Regarding Blastocystis sp. a total of 14 subtypes (ST1-ST5, ST7-ST11, ST13, ST15, ST19, ST39) have been identified in NHPs so far [34,58], while 16 subtypes (ST1-ST10, ST12, ST14, ST16, ST23, ST35, ST41) have been identified in humans [1,4,25,59]. The subtypes ST1-ST3 are the subtypes most frequently detected in both host groups [33,34]. In our study, the most common subtype was ST1 (57.1%), followed by ST3 (33%), ST2 (31.9%), ST8 (24.2%), ST5 (9.9%) and ST4 (1.1%). Similar results, with the predominance of the ST1 subtype, were also found in other studies investigating Blastocystis sp. in NHPs in European zoos [37,39,51], usually followed by the ST2 and ST3 subtypes [36,38,60]. These observations suggest that ST1, ST2 and ST3 subtypes are widespread in captive NHPs. We recorded subtype ST7, a typical bird subtype, in the caregiver from the Brno Zoo. Several such cases have been described in the past [12].
To our knowledge, our study is the first to employ an NGS approach to study Blastocystis sp. in NHPs. Next generation sequencing enables not only subtype identification, but also detection of mixed subtypes present within a sample [22,43]. However, the obtained results need to be interpreted carefully, as in our case, many of the mixed signals were unequal, with the minority subtype showing a very low sequencing signal - this could be explained not only by an animal excreting multiple subtypes in unequal proportions, but also by cross-contamination between feces collected from the cage floor. We recorded mixed colonization by multiple (two or three) subtypes in at least half of the qPCR-positive samples for Blastocystis sp. (50.5%). Mixed colonization of subtypes was detected in 33% of cases in the caregivers, specifically a combination of ST1 and ST3 from two zoos (Děčín Zoo, Dvůr Králové Zoo). In NHPs, mixed colonization of subtypes was observed in 52% of samples, with the combination of ST1 and ST3 (39%) being the most common, in all zoos except the Brno Zoo, specifically in the Cercopithecidae family. Previous studies that also reported a dominance of ST1 and ST3 subtypes in this family [51,61], but due to the different diagnostic methods they could not detect colonizations with mixed subtypes.
Other observed subtype combinations included ST2 and ST8, ST1 and ST2, ST1 and ST8, ST2 and ST3. A frequently occurred combination was the mixed colonization of ST2 and ST8 subtypes, found in 27% of mixed colonization cases in NHPs at the Olomouc Zoo, particularly in the family in Lemur catta and Eulemur macaco. Interestingly, the ST8 subtype was only detected in mixed colonization, contrary to previous findings by Stensvold et al. [62], where ST8 was primarily seen in the family Atelidae. The remaining mixed colonizations were observed in isolated cases. In Brno Zoo, despite a prevalence of 48.1% of Blastocystis sp., only one case of mixed colonization (ST1 + ST2) was found in Theropithecus gelada. Similarly, mixed colonization of ST1 and ST2 was found in a sample of Hylobates lar in Hodonín Zoo. In Děčín Zoo, a sample of Varecia rubra showed mixed colonization of ST1 and ST8, a combination that was also found in three samples of Nomascus gabriellae in Olomouc Zoo. There was also a sample of Symphalangus syndactylus at Olomouc Zoo that showed mixed colonization of ST2 and ST3. The isolated cases of mixed colonization in different zoos indicate that the specific conditions in individual zoos may influence the prevalence and combination of Blastocystis subtypes.
Despite extensive sampling, no mixed colonizations with Blastocystis subtypes were detected in Lemuridae in several zoos (Brno Zoo, Hodonín Zoo, Na Hrádečku Zoo). However, individual subtypes characteristic of this family (ST1, ST4, ST5, ST8) were identified. In particular, the detection of the ST5 subtype in Lemuridae at Brno Zoo, typically associated with pigs but previously found in NHPs, highlights the potential of the family to harbor a broad spectrum of Blastocystis subtypes, suggesting a large subtype variability within the Lemuridae.
Another interesting finding was the mixed colonization of three subtypes (ST1 + ST2 + ST3) in eight specimens of Macaca fuscata (family Cercopithecidae) at the Olomouc Zoo. Previous studies have already identified these subtypes in this group of macaques, but co-infections of these subtypes have not been reported so far [52,53], which opens a new perspective on Blastocystis colonization in these primates.
Our study addresses the issue of zoonotic transmission and highlights that Blastocystis sp. can be transmitted between NHPs and caregivers. We came across a striking case at the Děčín Zoo where a caregiver exhibited a mixed colonization (ST1 + ST3) mirroring the situation in seven NHPs under his/her care. This clearly indicates the possibility of zoonotic transmission. A similar scenario played out at Dvůr Králové Zoo, where a mixed colonization (ST1 + ST3) was detected in both the caregiver and three NHPs in his/her care, also indicating zoonotic transmission. Unfortunately, we could not further substantiate the potential for zoonotic transmission in these cases due to intra-subtype variability using the barcoding protocol (i.e. specific allele identification) as it is a mixed subtype colonization. However, these observations are noteworthy, given that this subtype combination is also common in humans [24,63,64].
In addition, the presence of an identical subtype and allele (ST1, allele 1) in both a caregiver and an NHP (Eulemur macaco) at the Olomouc Zoo suggests a possible transmission. This is supported by the higher prevalence of allele 1 of the ST1 subtype in NHPs similarly to other studies (e.g., [39]).
In Hodonín Zoo, although only one positive human sample was identified by qPCR, the low colonization intensity, indicated by a Ct value of 32, was below the threshold for subtype identification using the NGS approach, as described by Šloufová et al. [22]. The presence of Blastocystis sp. was confirmed based the short sequence from qPCR amplicon. Our results emphasize the urgent need for further research in this area to enhance our understanding of the dynamics governing zoonotic transmission of Blastocystis sp. between NHPs and humans.
Data from this study supports the designation of two new subtypes named ST47 and ST48. Two Blastocystis sp. nucleotide sequences from a chimpanzee in Brno Zoo were identified as likely novel based on their low sequence similarity with any known ST by NGS. Full-length SSU rRNA reference nucleotide sequences were obtained and analyzed to validate the identification of these novel sequences in accordance with current recommendations for Blastocystis nomenclature [4,26]. Pairwise distance and phylogenetic analyses indicate ST47 and ST48 vary from any accepted ST by at least 4%. These two new subtypes sit within a clade that contains ST1, ST2, ST11, and ST39. Except for ST11, which has largely been reported in elephants, this clade is composed of STs that are commonly reported in humans and NHPs, potentially supporting some degree of host preference within the clade. In addition to the analyses needed to describe these novel sequences as two new subtypes, we also conducted an analysis of the barcoding region of the SSU rRNA gene for the new ST sequences as it has been demonstrated that the conserved nature of this region makes it unsuitable for differentiation of all STs [26]. Based on this analysis we confirmed that ST47 has been previously described in chimpanzees from several other countries, but because the sequence analysis used in those previous studies relied upon only the barcoding region, these sequences were misclassified as ST1 (GenBank accession #s MZ182327, MZ496541, MZ496540, HQ286905, MN526862, MN526861, JQ974934). This is important to note for future studies on Blastocystis subtype diversity in humans and NHPs, as distinguishing subtypes within this clade cannot be performed using the barcoding region and will need to be performed using full-length reference sequences or the Santin region, and the sequence similarity needed for subtype designation will depend upon the region of the SSU rRNA gene being used for subtyping.
5. Conclusion
In conclusion, our study revealed that D. fragilis is only present in NHPs and not in caregivers, in contrast to Blastocystis sp. whose prevalence varies between zoos. Our results highlight the significant potential for zoonotic transmission of Blastocystis sp. between NHPs and their caregivers, as evidenced by shared subtypes and mixed colonization patterns. These findings align with the One Health concept, which emphasizes the mutual health of humans and animals. Furthermore, our findings reveal substantial genetic diversity within Blastocystis sp. including the identification of novel subtypes ST47 and ST48, expanding the known subtype spectrum in NHPs and emphasizing the need for comprehensive genetic studies. By using NGS, we were able to detect colonization with mixed subtypes in individual samples. However, these results should be interpreted with caution due to possible cross-contamination.
Author agreement
All authors have seen and approved the final version of the manuscript being submitted. We warrant that this article is our original work, has not received prior publication and is not under consideration for publication elsewhere.
Funding source
This work was financially supported by a grant from the Czech Science Foundation (22-04837S) to KJ. This publication is based upon work from COST Action CA21105, supported by COST (European Cooperation in Science and Technology). O.C. was financed by Ministry of Education, Youth and Sports of the Czech Republic, programme INTER-EXCELLENCE II, project LUC23165.
Ethical approval
The studies involving human participants were reviewed and approved by the Ethics Committee of the Biology Center of the Czech Academy of Sciences (reference number: 2/2020). Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin. All data were anonymized and processed according to the applicable laws of the Czech Republic (e.g., Act no. 101/2000 Coll and subsequent regulations).
Consent for publication
All caregivers signed the informed consent including consent for publication.
Availability of data and materials
The Blastocystis full-length nucleotide sequences obtained in this study have been deposited in GenBank under the accession numbers PP847211 and PP847212. Data from amplicon subtyping NGS are available in NCBI SRA database under accession number PRJNA1121083.
Authors contribution
Field work: AŠ, MK, KB, ZP, OK, KJP; Conceptualization: KJ, KJP, MJ, OK, ZP, KB, JM, MS; Methodology and investigation: AŠ, MK, ZP, KB, OK, KJP, MJ, OC, JM, MS; Data Curation: AŠ, MK, MJ, OC, JM, MS, KJ; Funding acquisition and project administration: KJ, OC; Software: AŠ, MK, MJ; ZP; KB; OC, JM; Validation and Visualization: AŠ, MK, MJ, ZP, OC, JM, MS, KJ; Writing – original draft: AŠ, MK, KJP, MJ, OC, JM, MS, KJ; Writing – review and editing: KJP, MJ, JM, MS, KJ. All authors contributed to the article and approved the submitted version.
CRediT authorship contribution statement
Anna Šejnohová: Writing – original draft, Visualization, Software, Methodology, Investigation, Data curation. Monika Koutenská: Writing – original draft, Visualization, Software, Methodology, Investigation, Data curation. Milan Jirků: Writing – review & editing, Writing – original draft, Visualization, Validation, Software, Methodology, Investigation, Data curation, Conceptualization. Kristýna Brožová: Software, Methodology, Investigation, Conceptualization. Zuzana Pavlíčková: Visualization, Validation, Software, Methodology, Investigation, Conceptualization. Oldřiška Kadlecová: Methodology, Investigation, Conceptualization. Ondřej Cinek: Writing – original draft, Visualization, Validation, Software, Methodology, Investigation, Funding acquisition, Data curation. Jenny G. Maloney: Writing – review & editing, Writing – original draft, Visualization, Validation, Software, Methodology, Investigation, Data curation, Conceptualization. Mónica Santín: Writing – review & editing, Writing – original draft, Visualization, Validation, Software, Methodology, Investigation, Data curation, Conceptualization. Klára J. Petrželková: Writing – review & editing, Writing – original draft, Methodology, Investigation, Conceptualization. Kateřina Jirků: Writing – review & editing, Writing – original draft, Validation, Supervision, Project administration, Methodology, Investigation, Funding acquisition, Data curation, Conceptualization.
Declaration of competing interest
Authors declare no conflict of interest.
Acknowledgements
The work could not be done without willingness and collaboration with participating zoos (Na Hrádečku Zoo, Hodonín Zoo, Děčín Zoo, Olomouc Zoo, Brno Zoo, and Dvůr Králové Zoo). We are grateful to all caregivers and primate curators, who provided us with samples of NHP and all caregivers who participated in the study themselves. We thank Aleksey Molokin and Nadja S. George for technical assistance.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.onehlt.2024.100862.
Contributor Information
Milan Jirků, Email: jirku@paru.cas.cz.
Kristýna Brožová, Email: kristyna.brozova@paru.cas.cz.
Zuzana Pavlíčková, Email: zuzana.lhotska@paru.cas.cz.
Oldřiška Kadlecová, Email: hlozkova@paru.cas.cz.
Ondřej Cinek, Email: Ondrej.Cinek@Lfmotol.cuni.cz.
Jenny G. Maloney, Email: jenny.maloney@usda.gov.
Mónica Santín, Email: monica.santin-duran@usda.gov.
Klára J. Petrželková, Email: petrzelkova@ivb.cz.
Kateřina Jirků, Email: pomajbikova@paru.cas.cz.
Appendix A. Supplementary data
Supplementary data 1. A comprehensive breakdown of the prevalence of Blastocystis sp. and Dientamoeba fragilis in the different primate families and suborders of the study. It includes the total number of samples taken from each family and suborder, along with the calculated prevalence rates for both protists.
Data availability
data are avalaible in GenBank databases - described in the MS
References
- 1.Stensvold C.R., Clark C.G. Current status of Blastocystis: a personal view. Parasitol. Int. 2016;65:763–771. doi: 10.1016/j.parint.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 2.Stark D., Barratt J., Chan D., Ellis J.T. Dientamoeba fragilis, the neglected trichomonad of the human bowel. Clin. Microbiol. Rev. 2016;29:553–580. doi: 10.1128/CMR.00076-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clark C.G., van der Giezen M., Alfellani M.A., Stensvold C.R. Recent developments in Blastocystis research. Adv. Parasitol. 2013;82:1–32. doi: 10.1016/B978-0-12-407706-5.00001-0. [DOI] [PubMed] [Google Scholar]
- 4.Stensvold C.R., Tan K.S.W., Clark C.G. Blastocystis. Trends Parasitol. 2020;36:315–316. doi: 10.1016/j.pt.2019.12.008. [DOI] [PubMed] [Google Scholar]
- 5.Stensvold C.R., van der Giezen M. Associations between gut microbiota and common luminal intestinal parasites. Trends Parasitol. 2018;34:369–377. doi: 10.1016/j.pt.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Billy V., Lhotská Z., Jirků M., et al. Blastocystis colonization alters the gut microbiome and, in some cases, promotes faster recovery from induced colitis. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.641483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deng L., Wojciech L., Gascoigne N.R.J., et al. New insights into the interactions between Blastocystis, the gut microbiota, and host immunity. PLoS Pathog. 2021;17 doi: 10.1371/JOURNAL.PPAT.1009253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jokelainen P., Jensen B.H., Andreassen B.U., et al. Dientamoeba fragilis, a commensal in children in Danish day care centers. J. Clin. Microbiol. 2017;55:1707–1713. doi: 10.1128/JCM.00037-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bart A., Wentink-Bonnema E.M.S., Gilis H., et al. Diagnosis and subtype analysis of Blastocystis sp. in 442 patients in a hospital setting in the Netherlands. BMC Infect. Dis. 2013;13:2–7. doi: 10.1186/1471-2334-13-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El Safadi D., Gaayeb L., Meloni D., et al. Children of Senegal River basin show the highest prevalence of Blastocystis sp. ever observed worldwide. BMC Infect. Dis. 2014;14:164. doi: 10.1186/1471-2334-14-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El Safadi D., Cian A., Nourrisson C., et al. Prevalence, risk factors for infection and subtype distribution of the intestinal parasite Blastocystis sp. from a large-scale multi-center study in France. BMC Infect. Dis. 2016;16:451. doi: 10.1186/s12879-016-1776-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lhotská Z., Jirků M., Hložková O., et al. A study on the prevalence and subtype diversity of the intestinal protist Blastocystis sp. in a gut-healthy human population in the Czech Republic. Front cell infect Microbiol. 2020;10:544335. doi: 10.3389/fcimb.2020.544335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leelayoova S., Rangsin R., Taamasri P., et al. Evidence of waterborne transmission of Blastocystis hominis. Am. J. Trop. Med. Hyg. 2004;70:658–662. doi: 10.4269/ajtmh.2004.70.658. [DOI] [PubMed] [Google Scholar]
- 14.Leelayoova S., Siripattanapipong S., Thathaisong U., et al. Drinking water: a possible source of Blastocystis spp. subtype 1 infection in schoolchildren of a rural community in Central Thailand. Am. J. Trop. Med. Hyg. 2008;79:401–406. doi: 10.4269/ajtmh.2008.79.401. [DOI] [PubMed] [Google Scholar]
- 15.Garcia L.S. Dientamoeba fragilis, one of the neglected intestinal protozoa. J. Clin. Microbiol. 2016;54:2243–2250. doi: 10.1128/JCM.00400-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clark C.G., Röser D., Stensvold C.R. Transmission of Dientamoeba fragilis: pinworm or cysts? Trends Parasitol. 2014;30:136–140. doi: 10.1016/j.pt.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 17.Röser D., Nejsum P., Carlsgart A.J., et al. DNA of Dientamoeba fragilis detected within surface-sterilized eggs of Enterobius vermicularis. Exp. Parasitol. 2013;133:57–61. doi: 10.1016/j.exppara.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 18.Ögren J., Dienus O., Löfgren S., et al. Dientamoeba fragilis DNA detection in Enterobius vermicularis eggs. Pathog Dis. 2013;69:157–158. doi: 10.1111/2049-632X.12071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Munasinghe V.S., Vella N.G.F., Ellis J.T., et al. Cyst formation and faecal-oral transmission of Dientamoeba fragilis - the missing link in the life cycle of an emerging pathogen. Int. J. Parasitol. 2013;43:879–883. doi: 10.1016/j.ijpara.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 20.Stark D., Garcia L.S., Barratt J.L.N., et al. Description of Dientamoeba fragilis cyst and precystic forms from human samples. J. Clin. Microbiol. 2014;52:2680–2683. doi: 10.1128/JCM.00813-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jirků M., Kašparová A., Lhotská Z., et al. A cross-sectional study on the occurrence of the intestinal protist, Dientamoeba fragilis, in the gut-healthy volunteers and their animals. Int. J. Mol. Sci. 2022;23:15407. doi: 10.3390/ijms232315407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Šloufová M., Lhotská Z., Jirků M., et al. Comparison of molecular diagnostic approaches for the detection and differentiation of the intestinal protist Blastocystis sp. in humans. Parasite. 2022;29:30. doi: 10.1051/parasite/2022029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maloney J.G., da Cunha M.J.R., Molokin A., et al. Next-generation sequencing reveals wide genetic diversity of Blastocystis subtypes in chickens including potentially zoonotic subtypes. Parasitol. Res. 2021;120:2219–2231. doi: 10.1007/s00436-021-07170-3. [DOI] [PubMed] [Google Scholar]
- 24.Hernández P.C., Maloney J.G., Molokin A., et al. Exploring Blastocystis genetic diversity in rural schoolchildren from Colombia using next-generation amplicon sequencing reveals significant associations between contact with animals and infection risk. Parasitol. Res. 2023;122:1451–1462. doi: 10.1007/s00436-023-07841-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maloney J.G., Molokin A., Segu R., et al. Identification and molecular characterization of four new Blastocystis subtypes designated ST35-ST38. Microorganisms. 2023;11:46. doi: 10.3390/microorganisms11010046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Santin M., Figueiredo A., Molokin A., et al. Division of Blastocystis ST10 into three new subtypes : ST42-ST44. J. Eukaryot. Microbiol. 2024;71 doi: 10.1111/jeu.12998. [DOI] [PubMed] [Google Scholar]
- 27.Koehler A.V., Herath H.M.P.D., Hall R.S., et al. Marked genetic diversity within Blastocystis in Australian wildlife revealed using a next generation sequencing–phylogenetic approach. Int J Parasitol Parasites Wildl. 2024;23 doi: 10.1016/j.ijppaw.2023.100902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stensvold C.R., Clark C.G., Röser D. Limited intra-genetic diversity in Dientamoeba fragilis housekeeping genes. Infect. Genet. Evol. 2013;18:284–286. doi: 10.1016/j.meegid.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 29.Windsor J.J., Clark C.G., Macfarlane L. Molecular typing of Dientamoeba fragilis. Br. J. Biomed. Sci. 2004;61:153–155. doi: 10.1080/09674845.2004.11978138. [DOI] [PubMed] [Google Scholar]
- 30.Johnson J.A., Clark C.G. Cryptic genetic diversity in Dientamoeba fragilis. J. Clin. Microbiol. 2000;38:4653–4654. doi: 10.1128/jcm.38.12.4653-4654.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peek R., Reedeker F.R., Van Gool T. Direct amplification and genotyping of Dientamoeba fragilis from human stool specimens. J. Clin. Microbiol. 2004;42:631–635. doi: 10.1128/JCM.42.2.631-635.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stensvold C.R., Clark C.G., Röser D. Limited intra-genetic diversity in Dientamoeba fragilis housekeeping genes. Infect. Genet. Evol. 2013;18:284–286. doi: 10.1016/j.meegid.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 33.Alfellani M.A., Stensvold C.R., Vidal-Lapiedra A., et al. Variable geographic distribution of Blastocystis subtypes and its potential implications. Acta Trop. 2013;126:11–18. doi: 10.1016/j.actatropica.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 34.Hublin J.S.Y., Maloney J.G., Santin M. Blastocystis in domesticated and wild mammals and birds. Res. Vet. Sci. 2021;135:260–282. doi: 10.1016/j.rvsc.2020.09.031. [DOI] [PubMed] [Google Scholar]
- 35.Yoshikawa H., Wu Z., Pandey K., et al. Molecular characterization of Blastocystis isolates from children and rhesus monkeys in Kathmandu. Nepal. Vet Parasitol. 2009;160:295–300. doi: 10.1016/j.vetpar.2008.11.029. [DOI] [PubMed] [Google Scholar]
- 36.Betts E.L., Gentekaki E., Tsaousis A.D. Exploring micro-eukaryotic diversity in the gut: co-occurrence of Blastocystis subtypes and other protists in zoo animals. Front. Microbiol. 2020;11:288. doi: 10.3389/fmicb.2020.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Köster P.C., Dashti A., Bailo B., et al. Occurrence and genetic diversity of protist parasites in captive non-human primates, zookeepers, and free-living sympatric rats in the córdoba zoo conservation Centre, Southern Spain. Animals. 2021;11:700. doi: 10.3390/ani11030700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rudzińska M., Kowalewska B., Waleron M., et al. Molecular characterization of Blastocystis from animals and their caregivers at the gdańsk zoo (Poland) and the assessment of zoonotic transmission. Biology (Basel) 2021;10:984. doi: 10.3390/biology10100984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Köster P.C., Martínez-Nevado E., González A., et al. Intestinal protists in captive non-human primates and their handlers in six European zoological gardens. Molecular Evidence of Zoonotic Transmission. Front Vet Sci. 2022;8 doi: 10.3389/fvets.2021.819887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Helenbrook W.D., Wade S.E., Shields W.M., et al. Gastrointestinal parasites of ecuadorian mantled howler monkeys (Alouatta palliata aequatorialis) based on fecal analysis. J. Parasitol. 2015;101:341–350. doi: 10.1645/13-356.1. [DOI] [PubMed] [Google Scholar]
- 41.Menu E., Davoust B., Mediannikov O., et al. Occurrence of ten protozoan enteric pathogens in three non-human primate populations. Pathogens. 2021;10:280. doi: 10.3390/pathogens10030280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stark D., Phillips O., Peckett D., et al. Gorillas are a host for Dientamoeba fragilis: an update on the life cycle and host distribution. Vet. Parasitol. 2008;151:21–26. doi: 10.1016/j.vetpar.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 43.Maloney J.G., Molokin A., Santin M. Next generation amplicon sequencing improves detection of Blastocystis mixed subtype infections. Infect. Genet. Evol. 2019;73:119–125. doi: 10.1016/j.meegid.2019.04.013. [DOI] [PubMed] [Google Scholar]
- 44.Edgar R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- 45.Scicluna S.M., Tawari B., Clark C.G. DNA barcoding of Blastocystis. Protist. 2006;157:77–85. doi: 10.1016/j.protis.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 46.Maloney J.G., Molokin A., Santin M. Use of Oxford Nanopore MinION to generate full - length sequences of the Blastocystis small subunit (SSU) rRNA gene. Parasit. Vectors. 2020;13:595. doi: 10.1186/s13071-020-04484-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980;16:111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- 48.Tamura K., Stecher G., Kumar S. MEGA11: molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021;38:3022–3027. doi: 10.1093/molbev/msab120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stark D., Beebe N., Marriott D., et al. Detection of Dientamoeba fragilis in fresh stool specimens using PCR. Int. J. Parasitol. 2005;35:57–62. doi: 10.1016/j.ijpara.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 50.De Quadros R.M., Weiss P.H.E., Marques S.M.T., Miletti L.C. Potential cross-contamination of similar Giardia duodenalis assemblage in children and pet dogs in southern Brazil, as determined by PCR-RFLP. Rev. Inst. Med. Trop. Sao Paulo. 2016;58:3–9. doi: 10.1590/S1678-9946201658066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cian A., El Safadi D., Osman M., et al. Molecular epidemiology of Blastocystis sp. in various animal groups from two French zoos and evaluation of potential zoonotic risk. PLoS One. 2017;12 doi: 10.1371/journal.pone.0169659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Valença-Barbosa C., Do Bomfim T.C.B., Teixeira B.R., et al. Molecular epidemiology of Blastocystis isolated from animals in the state of Rio de Janeiro, Brazil. PLoS One. 2019;14 doi: 10.1371/journal.pone.0210740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ma L., Qiao H., Wang H., et al. Molecular prevalence and subtypes of Blastocystis sp. in primates in northern China. Transbound. Emerg. Dis. 2020;67:2789–2796. doi: 10.1111/tbed.13644. [DOI] [PubMed] [Google Scholar]
- 54.Deng L., Yao J., Chen S., et al. First identification and molecular subtyping of Blastocystis sp. in zoo animals in southwestern China. Parasit. Vectors. 2021;14:11. doi: 10.1186/s13071-020-04515-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Köster P.C., Lapuente J., Pizarro A., et al. Presence and genetic diversity of enteric protists in captive and semi-captive non-human primates in côte d’Ivoire, Sierra Leone, and Peru. Int J Parasitol Parasites Wildl. 2022;17:26–34. doi: 10.1016/j.ijppaw.2021.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zanzani S.A., Gazzonis A.L., Epis S., Manfredi M.T. Study of the gastrointestinal parasitic fauna of captive non-human primates (Macaca fascicularis) Parasitol. Res. 2016;115:307–312. doi: 10.1007/s00436-015-4748-9. [DOI] [PubMed] [Google Scholar]
- 57.Lankester F., Kiyang J.A., Bailey W., Unwin S. Dientamoeba fragilis: initial evidence of pathogenicity in the western lowland gorilla (Gorilla gorilla gorilla) J. Zoo Wildl. Med. 2010;41:350–352. doi: 10.1638/2009-0190.1. [DOI] [PubMed] [Google Scholar]
- 58.Yu M., Yao Y., Xiao H., et al. Extensive prevalence and significant genetic differentiation of Blastocystis in high- and low-altitude populations of wild rhesus macaques in China. Parasit. Vectors. 2023;16:107. doi: 10.1186/s13071-023-05691-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hernández-Castro C., Maloney J.G., Agudelo-lópez S.P., et al. Identification and validation of novel Blastocystis subtype ST41 in a Colombian patient undergoing colorectal cancer screening. J. Eukaryot. Microbiol. 2023;70:el2978. doi: 10.1111/jeu.12978. [DOI] [PubMed] [Google Scholar]
- 60.Oliveira-Arbex AP, David ÉB, Tenório M da S, et al (2020) Diversity of Blastocystis subtypes in wild mammals from a zoo and two conservation units in southeastern Brazil. Infect. Genet. Evol. 78:104053. doi: 10.1016/j.meegid.2019.104053. [DOI] [PubMed]
- 61.Alfellani M.A., Jacob A.S., Perea N.O., et al. Diversity and distribution of Blastocystis sp. subtypes in non-human primates. Parasitology. 2013;140:966–971. doi: 10.1017/S0031182013000255. [DOI] [PubMed] [Google Scholar]
- 62.Stensvold C.R., Alfellani M.A., Nørskov-Lauritsen S., et al. Subtype distribution of Blastocystis isolates from synanthropic and zoo animals and identification of a new subtype. Int. J. Parasitol. 2009;39:473–479. doi: 10.1016/j.ijpara.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 63.Rojas-Velázquez L., Maloney J.G., Molokin A., et al. Use of next-generation amplicon sequencing to study Blastocystis genetic diversity in a rural human population from Mexico. Parasit. Vectors. 2019;12:566. doi: 10.1186/s13071-019-3814-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sarzhanov F., Dogruman-Al F., Santin M., et al. Investigation of neglected protists Blastocystis sp. and Dientamoeba fragilis in immunocompetent and immunodeficient diarrheal patients using both conventional and molecular methods. PLoS Negl. Trop. Dis. 2021;15 doi: 10.1371/JOURNAL.PNTD.0009779. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data 1. A comprehensive breakdown of the prevalence of Blastocystis sp. and Dientamoeba fragilis in the different primate families and suborders of the study. It includes the total number of samples taken from each family and suborder, along with the calculated prevalence rates for both protists.
Data Availability Statement
The Blastocystis full-length nucleotide sequences obtained in this study have been deposited in GenBank under the accession numbers PP847211 and PP847212. Data from amplicon subtyping NGS are available in NCBI SRA database under accession number PRJNA1121083.
data are avalaible in GenBank databases - described in the MS