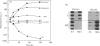Abstract
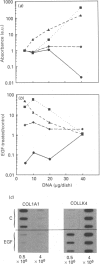
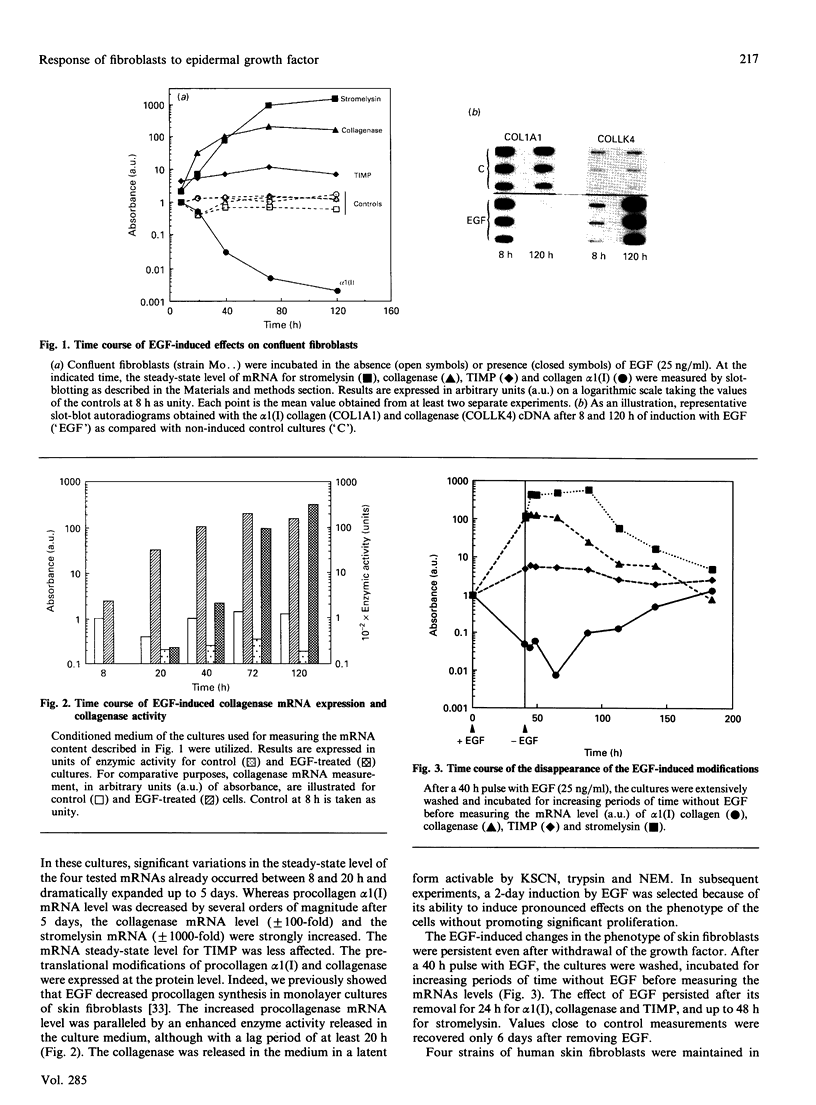
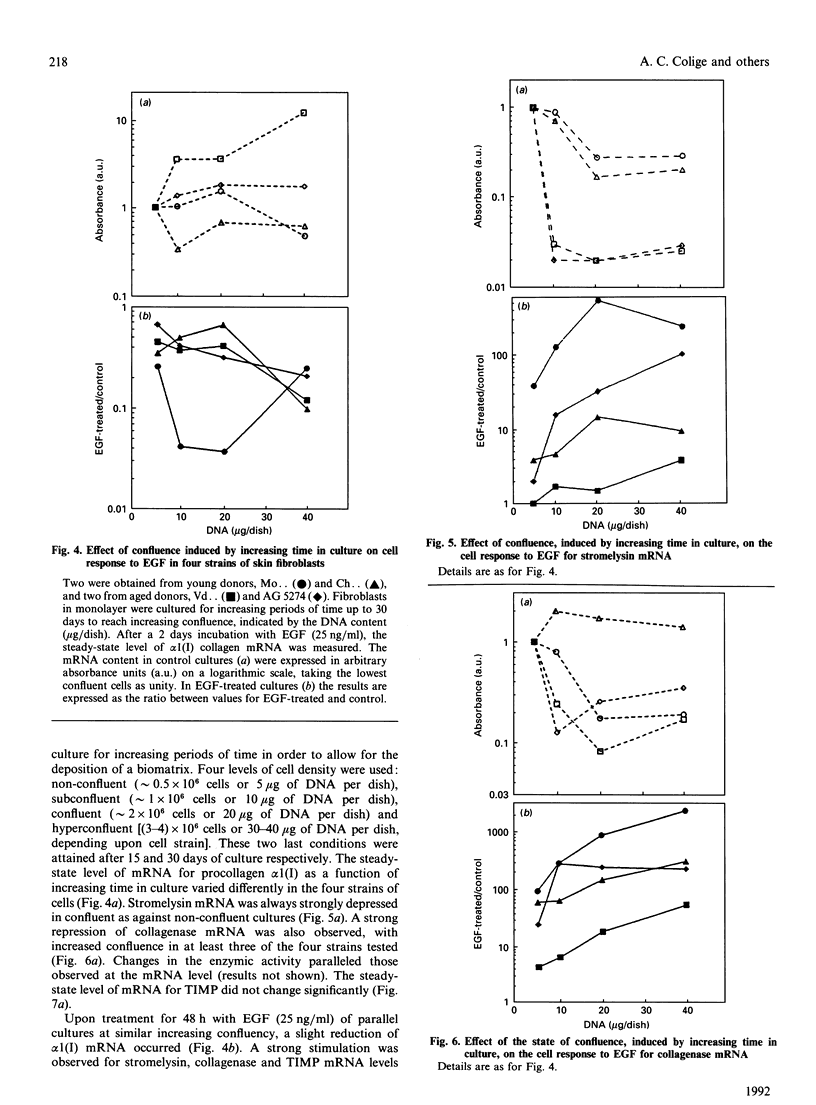
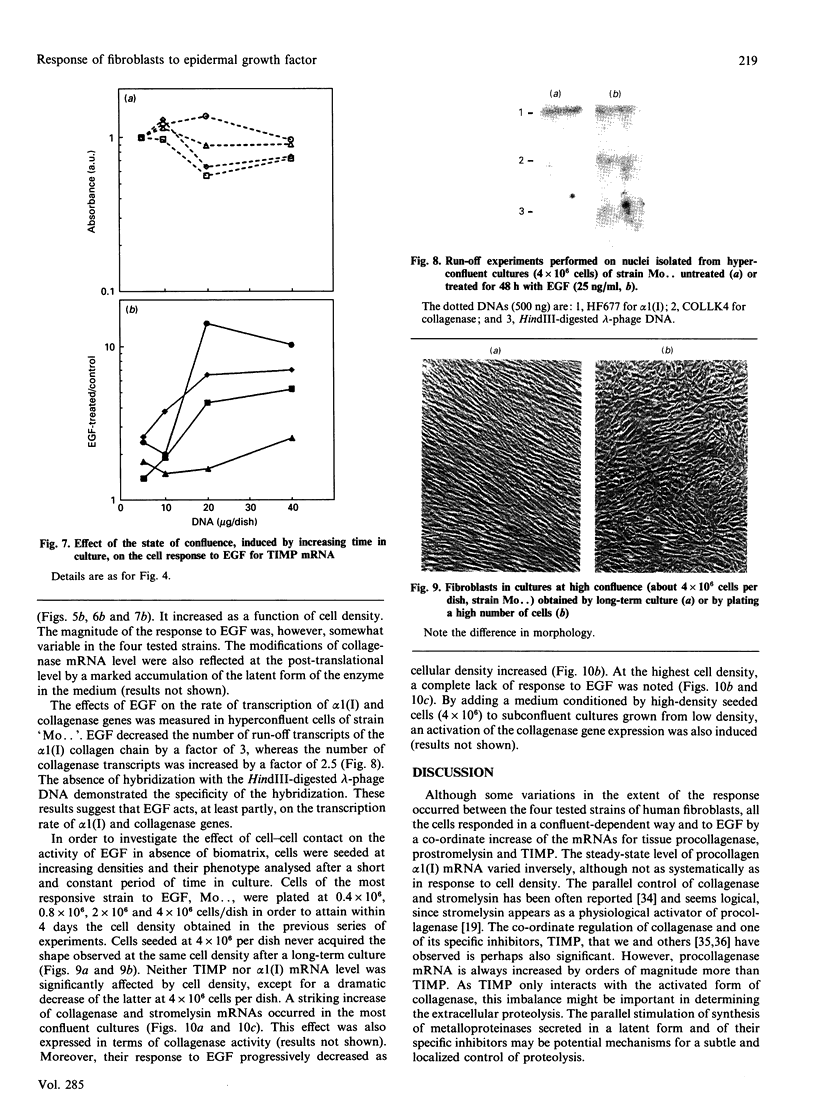
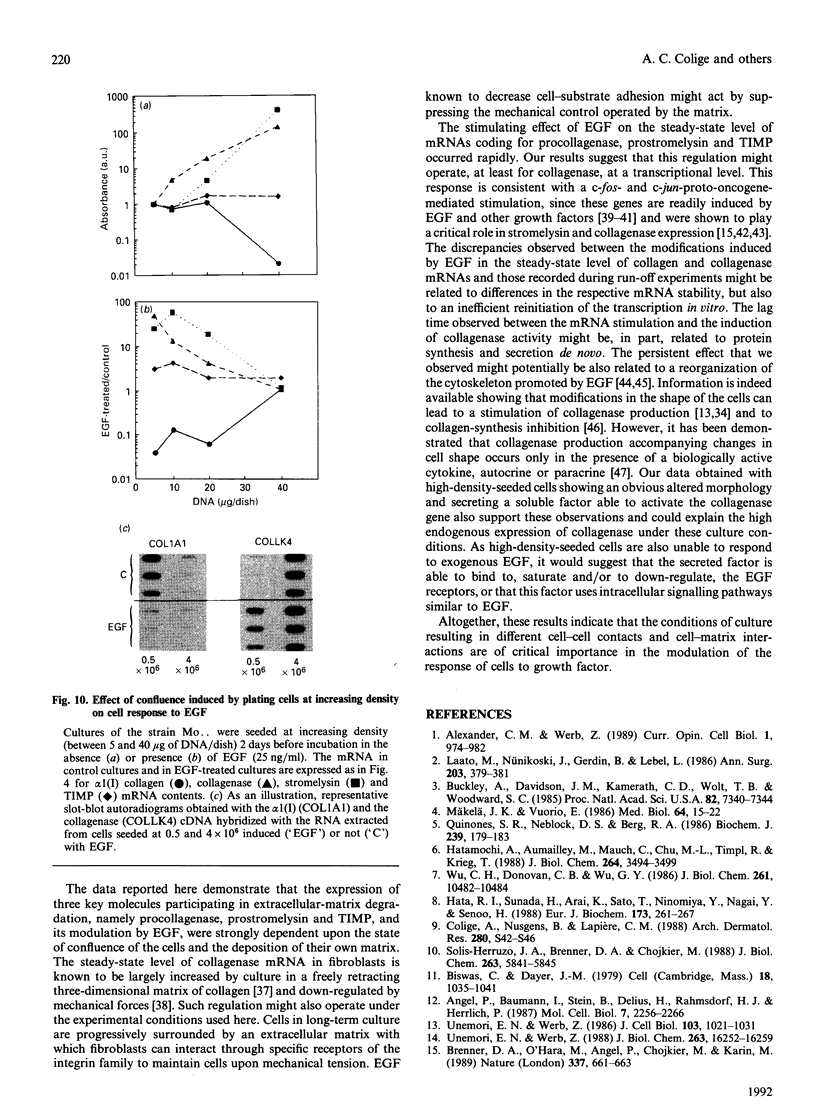
Investigations of the effect of epidermal growth factor (EGF) on the expression of four genes involved in the turnover of the extracellular matrix, collagen type I, collagenase, stromelysin and tissue inhibitor of metalloproteinases (TIMP) were performed on four strains of skin fibroblasts in vitro. Addition of EGF to subconfluent cultures for increasing periods of time up to 5 days induced an inhibition of procollagen alpha 1(I) mRNA and a strong stimulation of collagenase (100-fold) and stromelysin (1000-fold) mRNAs, whereas the mRNA of TIMP was increased to a lesser extent (5-fold). After a 40 h pulse with EGF, these effects persisted for 24-48 h after withdrawal of the growth factor and slowly diminished thereafter to attain control values after several days. By culturing fibroblasts for increasing periods of time, different levels of confluence were obtained allowing for the deposition of an extracellular biomatrix. The steady-state level of collagenase and stromelysin mRNAs were profoundly depressed in confluent as against non-confluent cultures, whereas no major change for TIMP and procollagen alpha 1(I) mRNAs was observed. Upon treatment of these cultures with EGF for 48h, the steady-state level of collagenase, stromelysin and TIMP increased, whereas procollagen alpha 1(I) mRNA was slightly reduced. These modifications were, at least in part, dependent upon a regulation of the transcription rate, as suggested from run-off experiments. Similar states of confluence were obtained by seeding cells at increasing densities in short-term cultures in which cell-cell contact predominated. In such culture conditions, the collagenase and stromelysin mRNAs were enhanced in high as compared to low density cultures. The response to EGF was progressively decreased for collagenase, stromelysin and, to a lesser extent, TIMP mRNAs at most densities and a complete lack of response to EGF at the highest cell density was observed. Under all culture conditions the modulation of collagenase mRNA was paralleled by similar modifications of enzyme activity. These results emphasize the importance of the cell-cell contacts and cell-matrix interactions in the expression of the genes coding for metalloproteinases or their inhibitor and their modulation by growth factors.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe S., Nagai Y. Interaction between tadpole collagenase and human 2 -macroglobulin. Biochim Biophys Acta. 1972 Aug 31;278(1):125–132. doi: 10.1016/0005-2795(72)90113-4. [DOI] [PubMed] [Google Scholar]
- Alexander C. M., Werb Z. Proteinases and extracellular matrix remodeling. Curr Opin Cell Biol. 1989 Oct;1(5):974–982. doi: 10.1016/0955-0674(89)90068-9. [DOI] [PubMed] [Google Scholar]
- Angel P., Baumann I., Stein B., Delius H., Rahmsdorf H. J., Herrlich P. 12-O-tetradecanoyl-phorbol-13-acetate induction of the human collagenase gene is mediated by an inducible enhancer element located in the 5'-flanking region. Mol Cell Biol. 1987 Jun;7(6):2256–2266. doi: 10.1128/mcb.7.6.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailly C., Drèze S., Asselineau D., Nusgens B., Lapière C. M., Darmon M. Retinoic acid inhibits the production of collagenase by human epidermal keratinocytes. J Invest Dermatol. 1990 Jan;94(1):47–51. doi: 10.1111/1523-1747.ep12873342. [DOI] [PubMed] [Google Scholar]
- Biswas C., Dayer J. M. Stimulation of collagenase production by collagen in mammalian cell cultures. Cell. 1979 Dec;18(4):1035–1041. doi: 10.1016/0092-8674(79)90216-2. [DOI] [PubMed] [Google Scholar]
- Bockus B. J., Stiles C. D. Regulation of cytoskeletal architecture by platelet-derived growth factor, insulin and epidermal growth factor. Exp Cell Res. 1984 Jul;153(1):186–197. doi: 10.1016/0014-4827(84)90460-9. [DOI] [PubMed] [Google Scholar]
- Brenner D. A., O'Hara M., Angel P., Chojkier M., Karin M. Prolonged activation of jun and collagenase genes by tumour necrosis factor-alpha. Nature. 1989 Feb 16;337(6208):661–663. doi: 10.1038/337661a0. [DOI] [PubMed] [Google Scholar]
- Buckley A., Davidson J. M., Kamerath C. D., Wolt T. B., Woodward S. C. Sustained release of epidermal growth factor accelerates wound repair. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7340–7344. doi: 10.1073/pnas.82.21.7340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chua C. C., Geiman D. E., Keller G. H., Ladda R. L. Induction of collagenase secretion in human fibroblast cultures by growth promoting factors. J Biol Chem. 1985 May 10;260(9):5213–5216. [PubMed] [Google Scholar]
- Colige A., Nusgens B., Lapiere C. M. Effect of EGF on human skin fibroblasts is modulated by the extracellular matrix. Arch Dermatol Res. 1988;280 (Suppl):S42–S46. [PubMed] [Google Scholar]
- Colige A., Nusgens B., Lapiere C. M. Response to epidermal growth factor of skin fibroblasts from donors of varying age is modulated by the extracellular matrix. J Cell Physiol. 1990 Dec;145(3):450–457. doi: 10.1002/jcp.1041450309. [DOI] [PubMed] [Google Scholar]
- Conca W., Kaplan P. B., Krane S. M. Increases in levels of procollagenase messenger RNA in cultured fibroblasts induced by human recombinant interleukin 1 beta or serum follow c-jun expression and are dependent on new protein synthesis. J Clin Invest. 1989 May;83(5):1753–1757. doi: 10.1172/JCI114077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delvoye P., Nusgens B., Lapière C. M. The capacity of retracting a collagen matrix is lost by dermatosparactic skin fibroblasts. J Invest Dermatol. 1983 Sep;81(3):267–270. doi: 10.1111/1523-1747.ep12518288. [DOI] [PubMed] [Google Scholar]
- Duncan M. R., Berman B. Differential regulation of collagen, glycosaminoglycan, fibronectin, and collagenase activity production in cultured human adult dermal fibroblasts by interleukin 1-alpha and beta and tumor necrosis factor-alpha and beta. J Invest Dermatol. 1989 May;92(5):699–706. doi: 10.1111/1523-1747.ep12696891. [DOI] [PubMed] [Google Scholar]
- Emonard H., Grimaud J. A. Matrix metalloproteinases. A review. Cell Mol Biol. 1990;36(2):131–153. [PubMed] [Google Scholar]
- Gisslow M. T., McBride B. C. A rapid sensitive collagenase assay. Anal Biochem. 1975 Sep;68(1):70–78. doi: 10.1016/0003-2697(75)90680-6. [DOI] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Hata R., Sunada H., Arai K., Sato T., Ninomiya Y., Nagai Y., Senoo H. Regulation of collagen metabolism and cell growth by epidermal growth factor and ascorbate in cultured human skin fibroblasts. Eur J Biochem. 1988 Apr 15;173(2):261–267. doi: 10.1111/j.1432-1033.1988.tb13993.x. [DOI] [PubMed] [Google Scholar]
- Hatamochi A., Aumailley M., Mauch C., Chu M. L., Timpl R., Krieg T. Regulation of collagen VI expression in fibroblasts. Effects of cell density, cell-matrix interactions, and chemical transformation. J Biol Chem. 1989 Feb 25;264(6):3494–3499. [PubMed] [Google Scholar]
- Heldin N. E., Westermark B. Epidermal growth factor, but not thyrotropin, stimulates the expression of c-fos and c-myc messenger ribonucleic acid in porcine thyroid follicle cells in primary culture. Endocrinology. 1988 Mar;122(3):1042–1046. doi: 10.1210/endo-122-3-1042. [DOI] [PubMed] [Google Scholar]
- Ito A., Nagase H. Evidence that human rheumatoid synovial matrix metalloproteinase 3 is an endogenous activator of procollagenase. Arch Biochem Biophys. 1988 Nov 15;267(1):211–216. doi: 10.1016/0003-9861(88)90025-2. [DOI] [PubMed] [Google Scholar]
- Kerr L. D., Holt J. T., Matrisian L. M. Growth factors regulate transin gene expression by c-fos-dependent and c-fos-independent pathways. Science. 1988 Dec 9;242(4884):1424–1427. doi: 10.1126/science.2462278. [DOI] [PubMed] [Google Scholar]
- Kuter I., Johnson-Wint B., Beaupre N., Gross J. Collagenase secretion accompanying changes in cell shape occurs only in the presence of a biologically active cytokine. J Cell Sci. 1989 Mar;92(Pt 3):473–485. doi: 10.1242/jcs.92.3.473. [DOI] [PubMed] [Google Scholar]
- Laato M., Niinikoski J., Lebel L., Gerdin B. Stimulation of wound healing by epidermal growth factor. A dose-dependent effect. Ann Surg. 1986 Apr;203(4):379–381. doi: 10.1097/00000658-198604000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Mauch C., Adelmann-Grill B., Hatamochi A., Krieg T. Collagenase gene expression in fibroblasts is regulated by a three-dimensional contact with collagen. FEBS Lett. 1989 Jul 3;250(2):301–305. doi: 10.1016/0014-5793(89)80743-4. [DOI] [PubMed] [Google Scholar]
- Mawatari M., Kohno K., Mizoguchi H., Matsuda T., Asoh K., Van Damme J., Welgus H. G., Kuwano M. Effects of tumor necrosis factor and epidermal growth factor on cell morphology, cell surface receptors, and the production of tissue inhibitor of metalloproteinases and IL-6 in human microvascular endothelial cells. J Immunol. 1989 Sep 1;143(5):1619–1627. [PubMed] [Google Scholar]
- Murphy G., Reynolds J. J., Werb Z. Biosynthesis of tissue inhibitor of metalloproteinases by human fibroblasts in culture. Stimulation by 12-O-tetradecanoylphorbol 13-acetate and interleukin 1 in parallel with collagenase. J Biol Chem. 1985 Mar 10;260(5):3079–3083. [PubMed] [Google Scholar]
- Mäkelä J. K., Vuorio E. Type I collagen messenger RNA levels in experimental granulation tissue and silicosis in rats. Med Biol. 1986;64(1):15–22. [PubMed] [Google Scholar]
- Nusgens B., Merrill C., Lapiere C., Bell E. Collagen biosynthesis by cells in a tissue equivalent matrix in vitro. Coll Relat Res. 1984 Oct;4(5):351–363. doi: 10.1016/s0174-173x(84)80003-5. [DOI] [PubMed] [Google Scholar]
- Paulsson Y., Bywater M., Heldin C. H., Westermark B. Effects of epidermal growth factor and platelet-derived growth factor on c-fos and c-myc mRNA levels in normal human fibroblasts. Exp Cell Res. 1987 Jul;171(1):186–194. doi: 10.1016/0014-4827(87)90261-8. [DOI] [PubMed] [Google Scholar]
- Quantin B., Breathnach R. Epidermal growth factor stimulates transcription of the c-jun proto-oncogene in rat fibroblasts. Nature. 1988 Aug 11;334(6182):538–539. doi: 10.1038/334538a0. [DOI] [PubMed] [Google Scholar]
- Quinones S. R., Neblock D. S., Berg R. A. Regulation of collagen production and collagen mRNA amounts in fibroblasts in response to culture conditions. Biochem J. 1986 Oct 1;239(1):179–183. doi: 10.1042/bj2390179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P. N., Belfall B., Duff C., Logan C., Kean V., Thompson M. W., Sylvester J. E., Gorski J. L., Schmickel R. D., Worton R. G. Cloning of the breakpoint of an X;21 translocation associated with Duchenne muscular dystrophy. Nature. 1985 Dec 19;318(6047):672–675. doi: 10.1038/318672a0. [DOI] [PubMed] [Google Scholar]
- Schlessinger J., Geiger B. Epidermal growth factor induces redistribution of actin and alpha-actinin in human epidermal carcinoma cells. Exp Cell Res. 1981 Aug;134(2):273–279. doi: 10.1016/0014-4827(81)90426-2. [DOI] [PubMed] [Google Scholar]
- Sellers A., Murphy G., Meikle M. C., Reynolds J. J. Rabbit bone collagenase inhibitor blocks the activity of other neutral metalloproteinases. Biochem Biophys Res Commun. 1979 Mar 30;87(2):581–587. doi: 10.1016/0006-291x(79)91834-5. [DOI] [PubMed] [Google Scholar]
- Solis-Herruzo J. A., Brenner D. A., Chojkier M. Tumor necrosis factor alpha inhibits collagen gene transcription and collagen synthesis in cultured human fibroblasts. J Biol Chem. 1988 Apr 25;263(12):5841–5845. [PubMed] [Google Scholar]
- Thompson K. L., Rosner M. R. Regulation of epidermal growth factor receptor gene expression by retinoic acid and epidermal growth factor. J Biol Chem. 1989 Feb 25;264(6):3230–3234. [PubMed] [Google Scholar]
- Unemori E. N., Werb Z. Collagenase expression and endogenous activation in rabbit synovial fibroblasts stimulated by the calcium ionophore A23187. J Biol Chem. 1988 Nov 5;263(31):16252–16259. [PubMed] [Google Scholar]
- Unemori E. N., Werb Z. Reorganization of polymerized actin: a possible trigger for induction of procollagenase in fibroblasts cultured in and on collagen gels. J Cell Biol. 1986 Sep;103(3):1021–1031. doi: 10.1083/jcb.103.3.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z., Hembry R. M., Murphy G., Aggeler J. Commitment to expression of the metalloendopeptidases, collagenase and stromelysin: relationship of inducing events to changes in cytoskeletal architecture. J Cell Biol. 1986 Mar;102(3):697–702. doi: 10.1083/jcb.102.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. H., Donovan C. B., Wu G. Y. Evidence for pretranslational regulation of collagen synthesis by procollagen propeptides. J Biol Chem. 1986 Aug 15;261(23):10482–10484. [PubMed] [Google Scholar]