Abstract
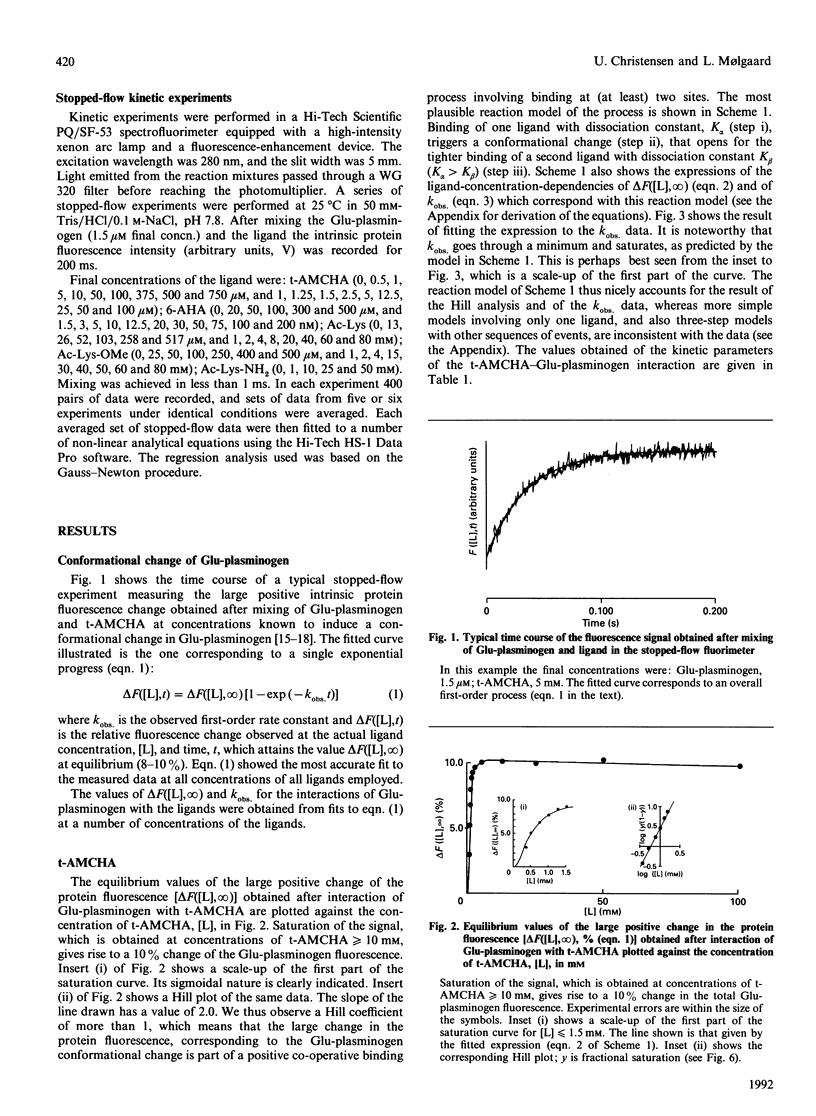
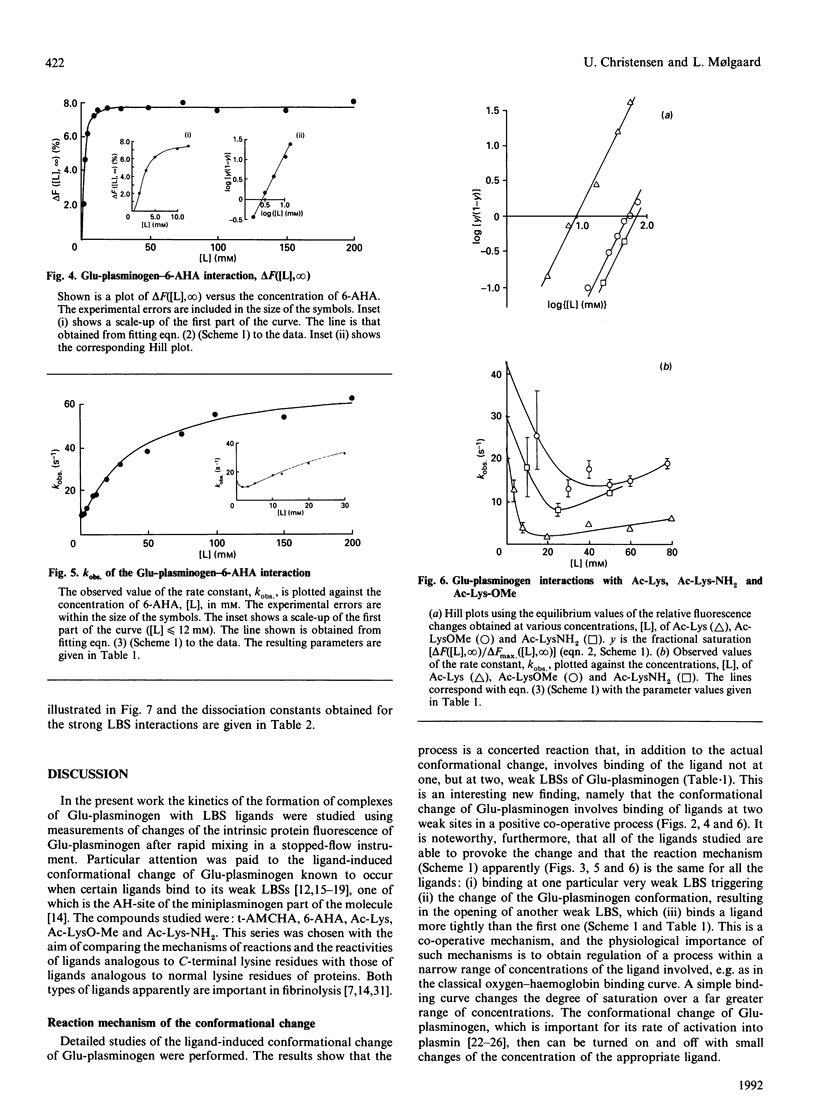
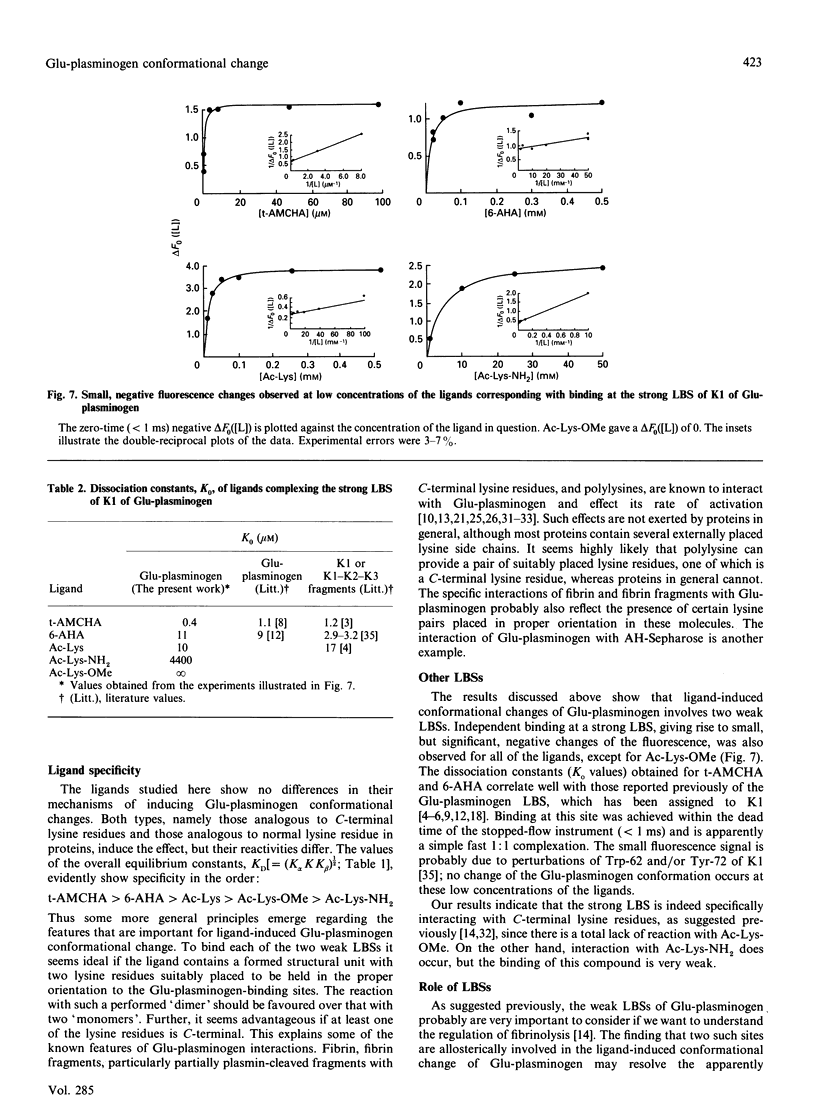
The kinetics of a series of Glu-plasminogen ligand-binding processes were investigated at pH 7.8 and 25 degrees C (in 0.1 M-NaCl). The ligands include compounds analogous to C-terminal lysine residues and to normal lysine residues. Changes of the Glu-plasminogen protein fluorescence were measured in a stopped-flow instrument as a function of time after rapid mixing of Glu-plasminogen and ligand at various concentrations. Large positive fluorescence changes (approximately 10%) accompany the ligand-induced conformational changes of Glu-plasminogen resulting from binding at weak lysine-binding sites. Detailed studies of the concentration-dependencies of the equilibrium signals and the rate constants of the process induced by various ligands showed the conformational change to involve two sites in a concerted positive co-operative process with three steps: (i) binding of a ligand at a very weak lysine-binding site that preferentially, but not exclusively, binds C-terminal-type lysine ligands, (ii) the rate-determining actual-conformational-change step and (iii) binding of one more lysine ligand at a second weak lysine-binding site that then binds the ligand more tightly. Further, totally independent initial small negative fluorescence changes (approximately 2-4%) corresponding to binding at the strong lysine-binding site of kringle 1 [Sottrup-Jensen, Claeys, Zajdel, Petersen & Magnusson (1978) Prog. Chem. Fibrinolysis Thrombolysis 3, 191-209] were observed for the C-terminal-type ligands. The finding that the conformational change in Glu-plasminogen involves two weak lysine-binding sites indicates that the effect cannot be assigned to any single kringle and that the problem of whether kringle 4 or kringle 5 is responsible for the process resolves itself. Probably kringle 4 and 5 are both participating. The involvement of two lysine binding-sites further makes the high specificity of Glu-plasminogen effectors more conceivable.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alkjaersig N. The purification and properties of human plasminogen. Biochem J. 1964 Oct;93(1):171–182. doi: 10.1042/bj0930171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen U. C-terminal lysine residues of fibrinogen fragments essential for binding to plasminogen. FEBS Lett. 1985 Mar 11;182(1):43–46. doi: 10.1016/0014-5793(85)81150-9. [DOI] [PubMed] [Google Scholar]
- Christensen U. Kinetic studies of the urokinase-catalysed conversion of NH2-terminal glutamic acid plasminogen to plasmin. Biochim Biophys Acta. 1977 Apr 12;481(2):638–647. doi: 10.1016/0005-2744(77)90297-2. [DOI] [PubMed] [Google Scholar]
- Christensen U., Mølgaard L. Stopped-flow fluorescence kinetic studies of Glu-plasminogen. Conformational changes triggered by AH-site ligand binding. FEBS Lett. 1991 Jan 28;278(2):204–206. doi: 10.1016/0014-5793(91)80117-l. [DOI] [PubMed] [Google Scholar]
- Christensen U. The AH-site of plasminogen and two C-terminal fragments. A weak lysine-binding site preferring ligands not carrying a free carboxylate function. Biochem J. 1984 Oct 15;223(2):413–421. doi: 10.1042/bj2230413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen U. Urokinase-catalysed plasminogen activation. Effects of ligands binding to the AH-site of plasminogen. Biochim Biophys Acta. 1988 Nov 23;957(2):258–265. doi: 10.1016/0167-4838(88)90281-6. [DOI] [PubMed] [Google Scholar]
- Deutsch D. G., Mertz E. T. Plasminogen: purification from human plasma by affinity chromatography. Science. 1970 Dec 4;170(3962):1095–1096. doi: 10.1126/science.170.3962.1095. [DOI] [PubMed] [Google Scholar]
- Hoylaerts M., Rijken D. C., Lijnen H. R., Collen D. Kinetics of the activation of plasminogen by human tissue plasminogen activator. Role of fibrin. J Biol Chem. 1982 Mar 25;257(6):2912–2919. [PubMed] [Google Scholar]
- Lerch P. G., Rickli E. E., Lergier W., Gillessen D. Localization of individual lysine-binding regions in human plasminogen and investigations on their complex-forming properties. Eur J Biochem. 1980;107(1):7–13. doi: 10.1111/j.1432-1033.1980.tb04617.x. [DOI] [PubMed] [Google Scholar]
- Lijnen H. R., Hoylaerts M., Collen D. Isolation and characterization of a human plasma protein with affinity for the lysine binding sites in plasminogen. Role in the regulation of fibrinolysis and identification as histidine-rich glycoprotein. J Biol Chem. 1980 Nov 10;255(21):10214–10222. [PubMed] [Google Scholar]
- Llinas M., De Marco A., Hochschwender S. M., Laursen R. A. A 1H-NMR study of isolated domains from human plasminogen. Structural homology between kringles 1 and 4. Eur J Biochem. 1983 Oct 3;135(3):379–391. doi: 10.1111/j.1432-1033.1983.tb07665.x. [DOI] [PubMed] [Google Scholar]
- Lucas M. A., Fretto L. J., McKee P. A. The binding of human plasminogen to fibrin and fibrinogen. J Biol Chem. 1983 Apr 10;258(7):4249–4256. [PubMed] [Google Scholar]
- Mangel W. F., Lin B. H., Ramakrishnan V. Characterization of an extremely large, ligand-induced conformational change in plasminogen. Science. 1990 Apr 6;248(4951):69–73. doi: 10.1126/science.2108500. [DOI] [PubMed] [Google Scholar]
- Markus G., DePasquale J. L., Wissler F. C. Quantitative determination of the binding of epsilon-aminocaproic acid to native plasminogen. J Biol Chem. 1978 Feb 10;253(3):727–732. [PubMed] [Google Scholar]
- Markus G., Evers J. L., Hobika G. H. Comparison of some properties of native (Glu) and modified (Lys) human plasminogen. J Biol Chem. 1978 Feb 10;253(3):733–739. [PubMed] [Google Scholar]
- Markus G., Priore R. L., Wissler F. C. The binding of tranexamic acid to native (Glu) and modified (Lys) human plasminogen and its effect on conformation. J Biol Chem. 1979 Feb 25;254(4):1211–1216. [PubMed] [Google Scholar]
- Matsuka Y. V., Novokhatny V. V., Kudinov S. A. Fluorescence spectroscopic analysis of ligand binding to kringle 1 + 2 + 3 and kringle 1 fragments from human plasminogen. Eur J Biochem. 1990 May 31;190(1):93–97. doi: 10.1111/j.1432-1033.1990.tb15550.x. [DOI] [PubMed] [Google Scholar]
- Motta A., Laursen R. A., Llinás M., Tulinsky A., Park C. H. Complete assignment of the aromatic proton magnetic resonance spectrum of the kringle 1 domain from human plasminogen: structure of the ligand-binding site. Biochemistry. 1987 Jun 30;26(13):3827–3836. doi: 10.1021/bi00387a014. [DOI] [PubMed] [Google Scholar]
- Motta A., Laursen R. A., Llinás M., Tulinsky A., Park C. H. Complete assignment of the aromatic proton magnetic resonance spectrum of the kringle 1 domain from human plasminogen: structure of the ligand-binding site. Biochemistry. 1987 Jun 30;26(13):3827–3836. doi: 10.1021/bi00387a014. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuizen W., Traas D. W. A rapid and simple method for the separation of four molecular forms of human plasminogen. Thromb Haemost. 1989 Apr 25;61(2):208–210. [PubMed] [Google Scholar]
- Peltz S. W., Hardt T. A., Mangel W. F. Positive regulation of activation of plasminogen by urokinase: differences in Km for (glutamic acid)-plasminogen and lysine-plasminogen and effect of certain alpha, omega-amino acids. Biochemistry. 1982 May 25;21(11):2798–2804. doi: 10.1021/bi00540a035. [DOI] [PubMed] [Google Scholar]
- Petersen L. C., Brender J., Suenson E. Zymogen-activation kinetics. Modulatory effects of trans-4-(aminomethyl)cyclohexane-1-carboxylic acid and poly-D-lysine on plasminogen activation. Biochem J. 1985 Jan 1;225(1):149–158. doi: 10.1042/bj2250149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickli E. E., Otavsky W. I. A new method of isolation and some properties of the heavy chain of human plasmin. Eur J Biochem. 1975 Nov 15;59(2):441–447. doi: 10.1111/j.1432-1033.1975.tb02472.x. [DOI] [PubMed] [Google Scholar]
- Rákóczi I., Wiman B., Collen D. On the biological significance of the specific interaction between fibrin, plasminogen and antiplasmin. Biochim Biophys Acta. 1978 May 3;540(2):295–300. doi: 10.1016/0304-4165(78)90142-3. [DOI] [PubMed] [Google Scholar]
- Rånby M. Studies on the kinetics of plasminogen activation by tissue plasminogen activator. Biochim Biophys Acta. 1982 Jun 24;704(3):461–469. doi: 10.1016/0167-4838(82)90068-1. [DOI] [PubMed] [Google Scholar]
- Suenson E., Lützen O., Thorsen S. Initial plasmin-degradation of fibrin as the basis of a positive feed-back mechanism in fibrinolysis. Eur J Biochem. 1984 May 2;140(3):513–522. doi: 10.1111/j.1432-1033.1984.tb08132.x. [DOI] [PubMed] [Google Scholar]
- Thewes T., Constantine K., Byeon I. J., Llinás M. Ligand interactions with the kringle 5 domain of plasminogen. A study by 1H NMR spectroscopy. J Biol Chem. 1990 Mar 5;265(7):3906–3915. [PubMed] [Google Scholar]
- Thorsen S. Differences in the binding to fibrin of native plasminogen and plasminogen modified by proteolytic degradation. Influence of omega-aminocarboxylic acids. Biochim Biophys Acta. 1975 May 30;393(1):55–65. doi: 10.1016/0005-2795(75)90216-0. [DOI] [PubMed] [Google Scholar]
- Thorsen S., Kok P., Astrup T. Reversible and irreversible alterations of human plasminogen indicated by changes in susceptibility to plasminogen activators and in response to epsilon-aminocaproic acid. Thromb Diath Haemorrh. 1974 Dec 31;32(2-3):325–340. [PubMed] [Google Scholar]
- Trexler M., Váli Z., Patthy L. Structure of the omega-aminocarboxylic acid-binding sites of human plasminogen. Arginine 70 and aspartic acid 56 are essential for binding of ligand by kringle 4. J Biol Chem. 1982 Jul 10;257(13):7401–7406. [PubMed] [Google Scholar]
- Violand B. N., Byrne R., Castellino F. J. The effect of alpha-,omega-amino acids on human plasminogen structure and activation. J Biol Chem. 1978 Aug 10;253(15):5395–5401. [PubMed] [Google Scholar]
- Váli Z., Patthy L. Location of the intermediate and high affinity omega-aminocarboxylic acid-binding sites in human plasminogen. J Biol Chem. 1982 Feb 25;257(4):2104–2110. [PubMed] [Google Scholar]
- Wiman B., Collen D. Molecular mechanism of physiological fibrinolysis. Nature. 1978 Apr 6;272(5653):549–550. doi: 10.1038/272549a0. [DOI] [PubMed] [Google Scholar]
- Wiman B., Lijnen H. R., Collen D. On the specific interaction between the lysine-binding sites in plasmin and complementary sites in alpha2-antiplasmin and in fibrinogen. Biochim Biophys Acta. 1979 Jul 25;579(1):142–154. doi: 10.1016/0005-2795(79)90094-1. [DOI] [PubMed] [Google Scholar]


