Figure 4.
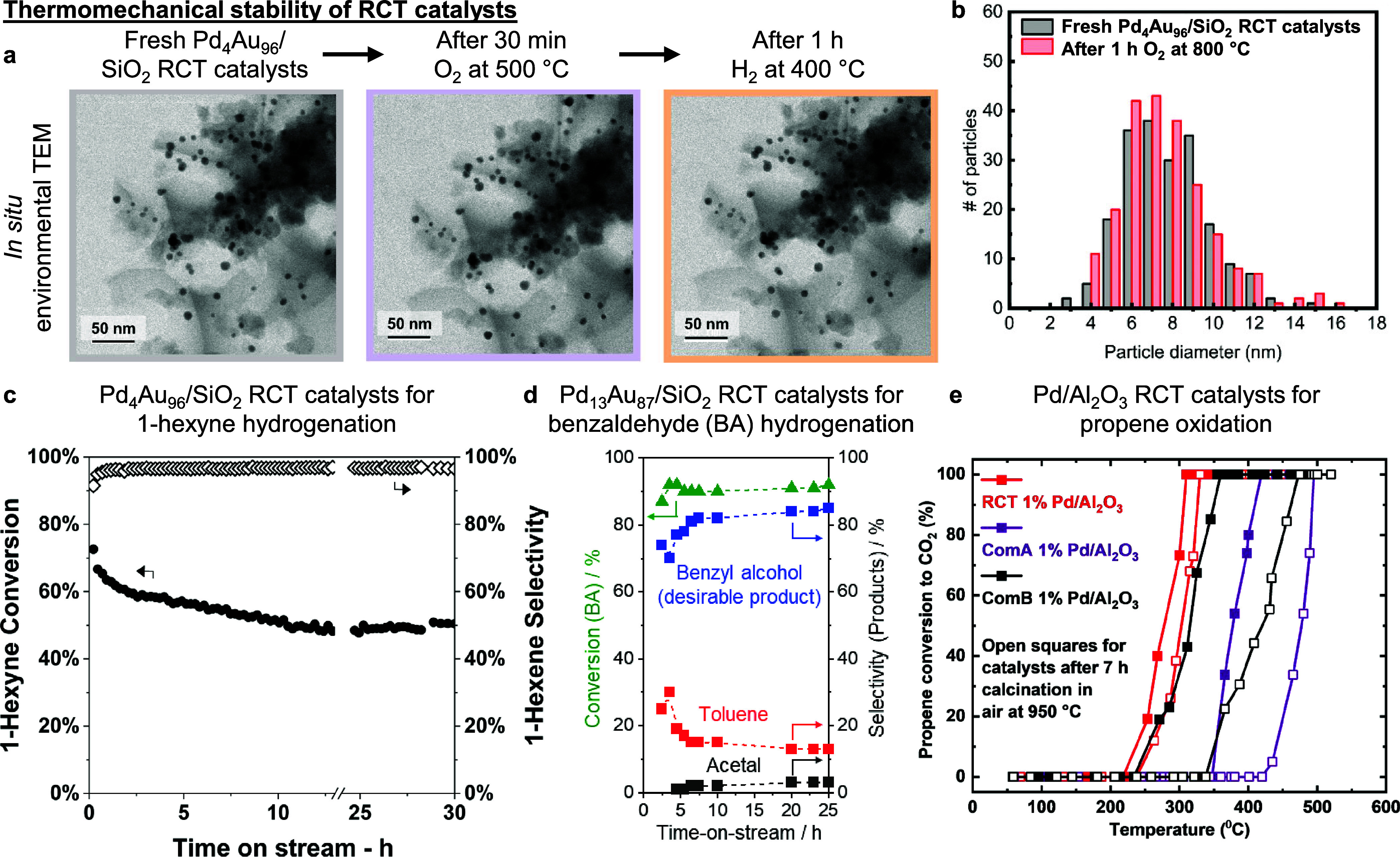
High thermomechanical stability of RCT catalysts. (a) In situ environmental transmission electron microscopy images depicting the intact macroporous SiO2 RCT structure and absence of NP migration after high temperature O2 and then H2 treatment. (b) Preserved NP size distribution in Pd4Au96/SiO2 RCT catalysts after 1 h of O2 treatment at 800 °C, indicating no appreciable NP sintering. The high thermomechanical stability of RCT catalysts allows for the high levels of catalytic activity and selectivity to be maintained in the continuous hydrogenation of (c) 1-hexyne to 1-hexene (gas phase), and (d) benzaldehyde to benzyl alcohol (liquid phase). (e) When tested for propene oxidation, Pd/Al2O3 RCT catalysts record comparable light-off curves before (filled red squares) and after 7 h of calcination in air at 950 °C (open red squares). For comparison, two different commercial Pd/Al2O3 catalysts (labeled ComA and ComB) require appreciably higher temperatures to achieve their precalcination conversion levels due to severe NP aggregation and sintering postcalcination. Data of catalysts before and after calcination in (d) are shown with filled and open squares, respectively. (a) adapted with permission from ref (100). Copyright 2020 The Authors under a CC-BY 4.0 license. (b) adapted with permission from ref (47). Copyright 2021 Wiley-VCH. (c) adapted with permission from ref (98). Copyright 2019 American Chemical society. (d) adapted with permission from ref (89). Copyright 2023 American Chemical society. (e) adapted with permission from ref (91). Copyright 2020 Elsevier B.V.
