Figure 6.
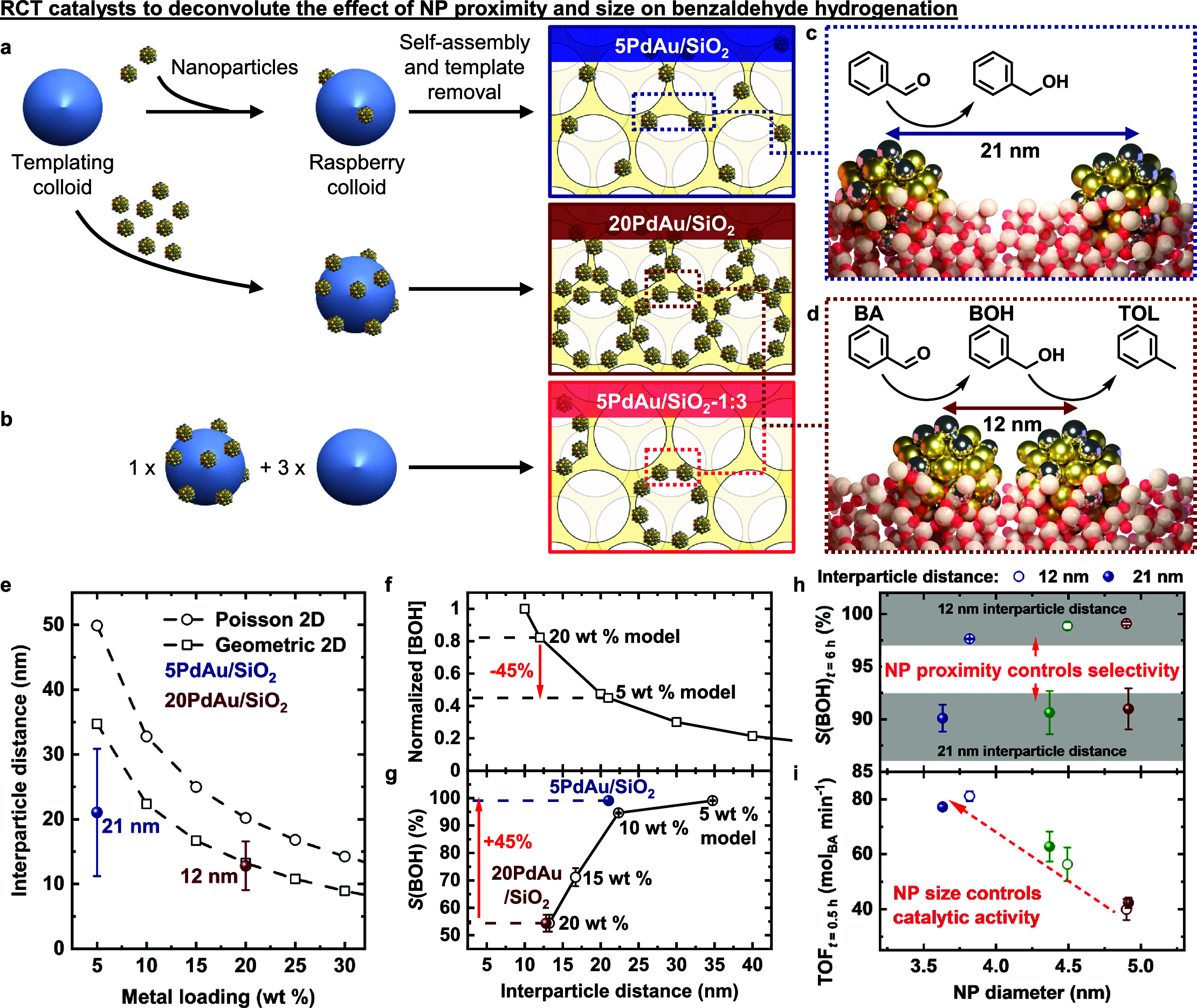
Effect of NP proximity and size on benzaldehyde hydrogenation using RCT catalysts. (a) Tuning the average interparticle distance from 21 to 12 nm by increasing the metal loading from 5 to 20 wt %, which in turn is achieved by increasing the NP to templating colloid ratio by a factor of 4 while maintaining the same amount of templating colloids added. xPdAu/SiO2 refer to x wt % Pd12Au88/SiO2 RCT catalysts. (b) To decouple the metal loading from interparticle distance, NP-decorated raspberry colloids (corresponding to 20 wt %) were mixed with NP-free templating colloids in a 1:3 ratio in the self-assembly step to achieve an overall metal loading of 5 wt % for comparison with 5PdAu/SiO2 (at the same metal loading) and 20PdAu/SiO2 (at the same interparticle distance). Effect of (c) larger and (d) smaller interparticle distance on benzaldehyde hydrogenation. BA, BOH, and TOL refer to benzaldehyde, benzyl alcohol, and toluene, respectively. (e) Average interparticle distance measured experimentally, compared to a 2D statistical (Poisson) and 2D geometrical model. As a function of interparticle distance, (f) COMSOL-simulated local BOH concentration ([BOH]) in between NPs and (g) the experimentally measured selectivity to BOH. As a function of NP size, (h) experimentally measured selectivity to BOH (S(BOH)) and (i) turnover frequency (TOF) of benzaldehyde. In (h)–(i), catalysts prepared at larger (21 nm) and smaller (12 nm) interparticle distances are shown with filled and open circles, respectively. (a)–(g) adapted with permission from ref (23). Copyright 2024 Springer Nature. (h)–(i) adapted with permission from ref (96). Copyright 2024 American Chemical Society.
