Abstract
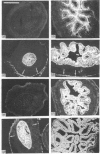
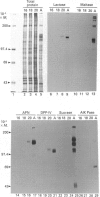
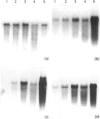
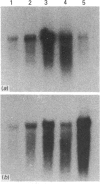
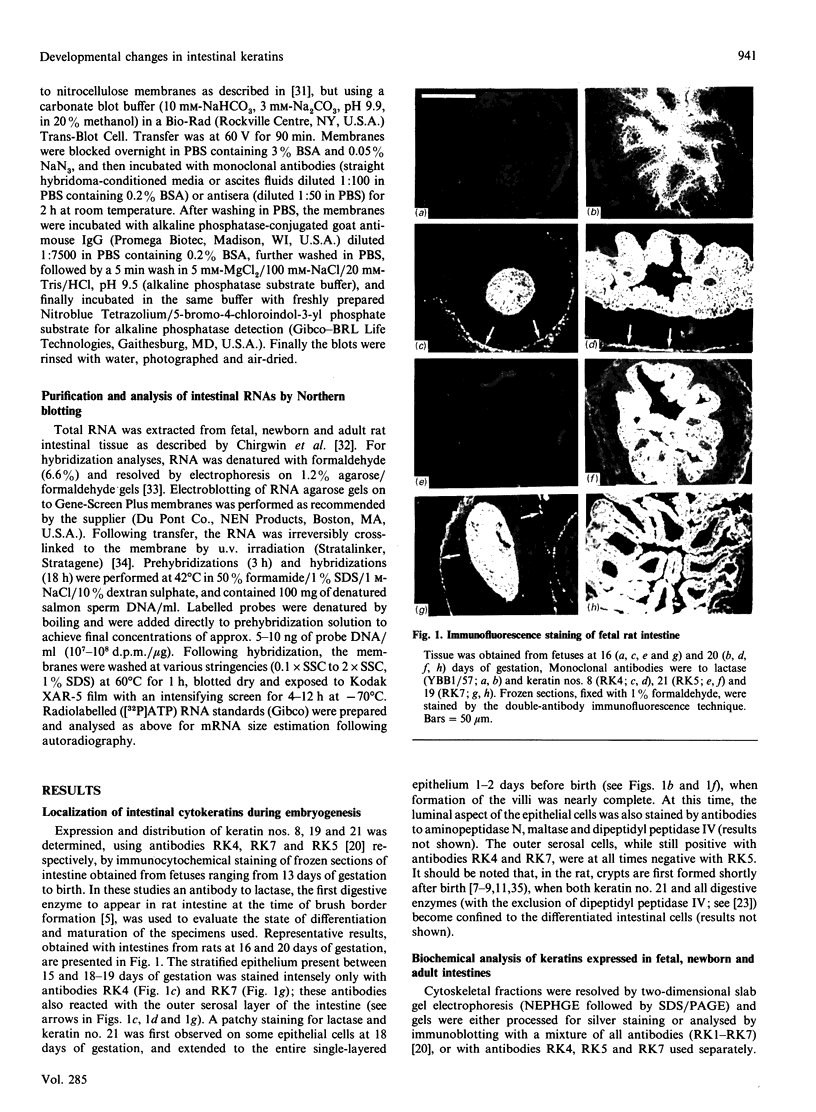
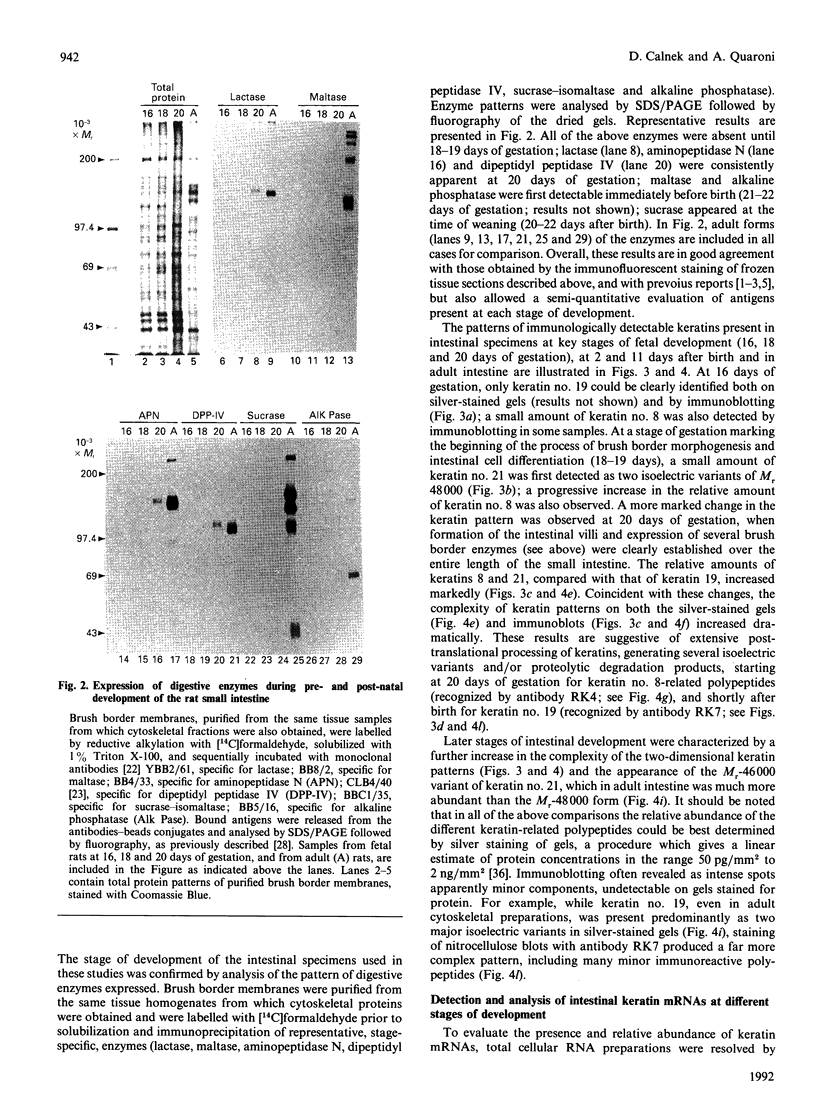
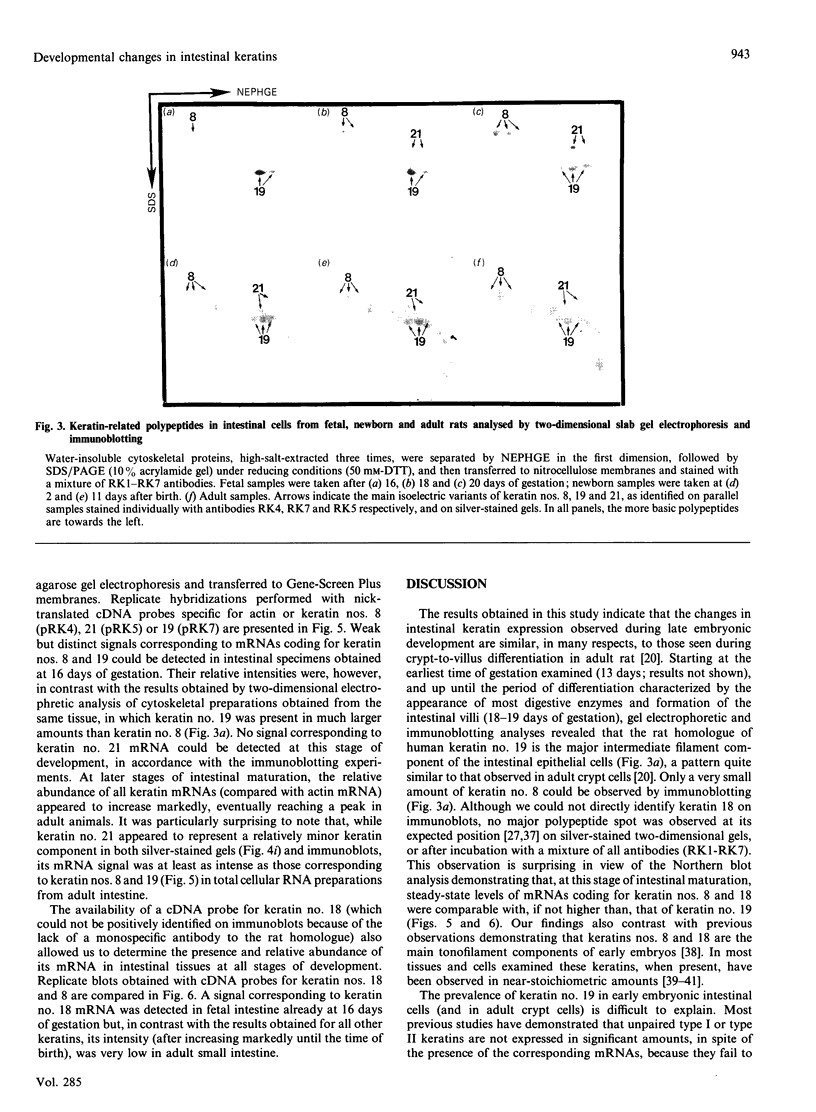
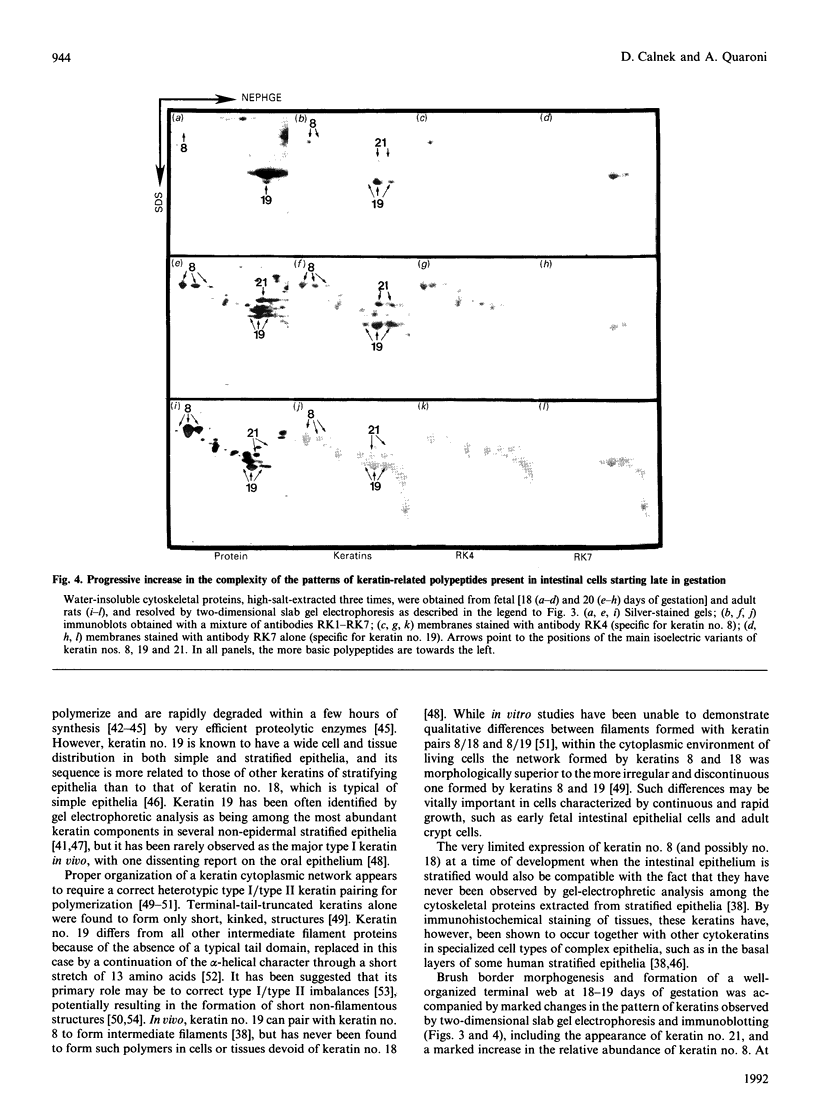
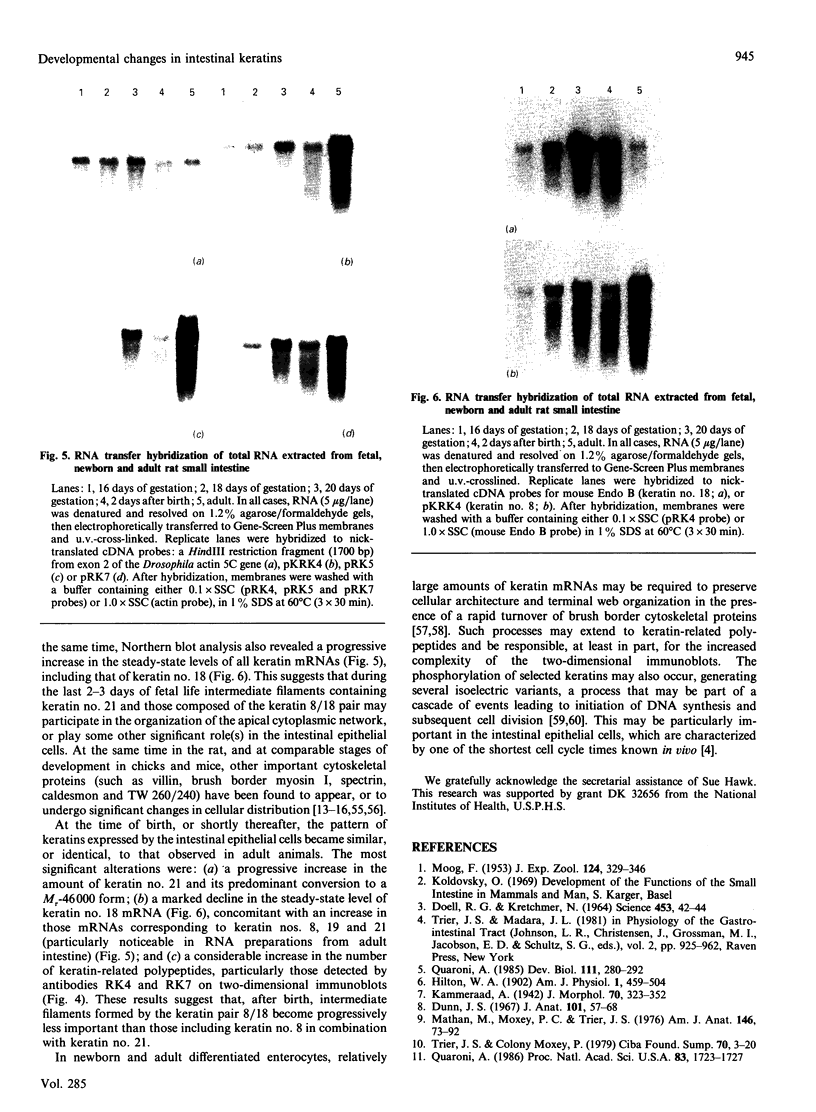
We have investigated keratin expression in fetal, newborn and adult rat intestines by immunofluorescence staining, immunoblotting of two-dimensional gels and Northern blot analysis of total cellular RNAs. Keratin-type intermediate filaments, composed predominantly of keratin no. 19, were observed already in the undifferentiated stratified epithelium present at 15-16 days of gestation. The marked maturation and differentiation of the epithelium taking place at 18-19 days of gestation was characterized by the appearance of the differentiation-specific keratin no. 21 and by a significant increase in the relative amount of keratin no. 8. The keratin pattern typical of adult villus cells became established at the time of birth, and was marked by a considerable increase in the complexity of the keratin-related polypeptides detected on two-dimensional gels, indicative of extensive post-translational modification of all keratins. Starting at 20 days of gestation there was a major increase in the relative abundance of mRNAs coding for keratin nos. 8, 19 and 21; in contrast, the relative amount of keratin no. 18 mRNA reached a peak shortly after birth and declined to very low levels in adult intestine. These results demonstrated marked changes in keratin expression and post-translational processing taking place at key stages of intestinal development. The appearance of keratin no. 21 in coincidence with the formation of an adult-type brush border and terminal web would be consistent with it having an important role in the organization of the intermediate filament network in the apical cytoplasm of the differentiated intestinal cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bader B. L., Magin T. M., Hatzfeld M., Franke W. W. Amino acid sequence and gene organization of cytokeratin no. 19, an exceptional tail-less intermediate filament protein. EMBO J. 1986 Aug;5(8):1865–1875. doi: 10.1002/j.1460-2075.1986.tb04438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baribault H., Blouin R., Bourgon L., Marceau N. Epidermal growth factor-induced selective phosphorylation of cultured rat hepatocyte 55-kD cytokeratin before filament reorganization and DNA synthesis. J Cell Biol. 1989 Oct;109(4 Pt 1):1665–1676. doi: 10.1083/jcb.109.4.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch F. X., Leube R. E., Achtstätter T., Moll R., Franke W. W. Expression of simple epithelial type cytokeratins in stratified epithelia as detected by immunolocalization and hybridization in situ. J Cell Biol. 1988 May;106(5):1635–1648. doi: 10.1083/jcb.106.5.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celis J. E., Larsen P. M., Fey S. J., Celis A. Phosphorylation of keratin and vimentin polypeptides in normal and transformed mitotic human epithelial amnion cells: behavior of keratin and vimentin filaments during mitosis. J Cell Biol. 1983 Nov;97(5 Pt 1):1429–1434. doi: 10.1083/jcb.97.5.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers C., Grey R. D. Development of the structural components of the brush border in absorptive cells of the chick intestine. Cell Tissue Res. 1979;204(3):387–405. doi: 10.1007/BF00233651. [DOI] [PubMed] [Google Scholar]
- Chandler J. S., Calnek D., Quaroni A. Identification and characterization of rat intestinal keratins. Molecular cloning of cDNAs encoding cytokeratins 8, 19, and a new 49-kDa type I cytokeratin (cytokeratin 21) expressed by differentiated intestinal epithelial cells. J Biol Chem. 1991 Jun 25;266(18):11932–11938. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowell G. M., Danielsen E. M. Biosynthesis of intestinal microvillar proteins. Rapid expression of cytoskeletal components in microvilli of pig small intestinal mucosal explants. FEBS Lett. 1984 Jul 9;172(2):309–314. doi: 10.1016/0014-5793(84)81147-3. [DOI] [PubMed] [Google Scholar]
- DOELL R. G., KRETCHMER N. INTESTINAL INVERTASE: PRECOCIOUS DEVELOPMENT OF ACTIVITY AFTER INJECTION OF HYDROCORTISONE. Science. 1964 Jan 3;143(3601):42–44. doi: 10.1126/science.143.3601.42. [DOI] [PubMed] [Google Scholar]
- Domenjoud L., Jorcano J. L., Breuer B., Alonso A. Synthesis and fate of keratins 8 and 18 in nonepithelial cells transfected with cDNA. Exp Cell Res. 1988 Dec;179(2):352–361. doi: 10.1016/0014-4827(88)90274-1. [DOI] [PubMed] [Google Scholar]
- Dunn J. S. The fine structure of the absorptive epithelial cells of the developing small intestine of the rat. J Anat. 1967 Jan;101(Pt 1):57–68. [PMC free article] [PubMed] [Google Scholar]
- Eckert R. L. Sequence of the human 40-kDa keratin reveals an unusual structure with very high sequence identity to the corresponding bovine keratin. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1114–1118. doi: 10.1073/pnas.85.4.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichner R., Sun T. T., Aebi U. The role of keratin subfamilies and keratin pairs in the formation of human epidermal intermediate filaments. J Cell Biol. 1986 May;102(5):1767–1777. doi: 10.1083/jcb.102.5.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzell R. M., Chafel M. M., Matsudaira P. T. Differential localization of villin and fimbrin during development of the mouse visceral endoderm and intestinal epithelium. Development. 1989 Jun;106(2):407–419. doi: 10.1242/dev.106.2.407. [DOI] [PubMed] [Google Scholar]
- Fath K. R., Obenauf S. D., Burgess D. R. Cytoskeletal protein and mRNA accumulation during brush border formation in adult chicken enterocytes. Development. 1990 Jun;109(2):449–459. doi: 10.1242/dev.109.2.449. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Appelhans B., Schmid E., Freudenstein C., Osborn M., Weber K. The organization of cytokeratin filaments in the intestinal epithelium. Eur J Cell Biol. 1979 Aug;19(3):255–268. [PubMed] [Google Scholar]
- Franke W. W., Denk H., Kalt R., Schmid E. Biochemical and immunological identification of cytokeratin proteins present in hepatocytes of mammalian liver tissue. Exp Cell Res. 1981 Feb;131(2):299–318. doi: 10.1016/0014-4827(81)90234-2. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Winter S., Grund C., Schmid E., Schiller D. L., Jarasch E. D. Isolation and characterization of desmosome-associated tonofilaments from rat intestinal brush border. J Cell Biol. 1981 Jul;90(1):116–127. doi: 10.1083/jcb.90.1.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z., Mackenzie I. C., Williams D. M., Cruchley A. T., Leigh I., Lane E. B. Patterns of keratin-expression in rests of Malassez and periapical lesions. J Oral Pathol. 1988 Apr;17(4):178–185. doi: 10.1111/j.1600-0714.1988.tb01521.x. [DOI] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Glenney P. Spectrin, fodrin, and TW260/240: a family of related proteins lining the plasma membrane. Cell Motil. 1983;3(5-6):671–682. doi: 10.1002/cm.970030531. [DOI] [PubMed] [Google Scholar]
- Hatzfeld M., Franke W. W. Pair formation and promiscuity of cytokeratins: formation in vitro of heterotypic complexes and intermediate-sized filaments by homologous and heterologous recombinations of purified polypeptides. J Cell Biol. 1985 Nov;101(5 Pt 1):1826–1841. doi: 10.1083/jcb.101.5.1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzfeld M., Weber K. The coiled coil of in vitro assembled keratin filaments is a heterodimer of type I and II keratins: use of site-specific mutagenesis and recombinant protein expression. J Cell Biol. 1990 Apr;110(4):1199–1210. doi: 10.1083/jcb.110.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heintzelman M. B., Mooseker M. S. Assembly of the brush border cytoskeleton: changes in the distribution of microvillar core proteins during enterocyte differentiation in adult chicken intestine. Cell Motil Cytoskeleton. 1990;15(1):12–22. doi: 10.1002/cm.970150104. [DOI] [PubMed] [Google Scholar]
- Heintzelman M. B., Mooseker M. S. Structural and compositional analysis of early stages in microvillus assembly in the enterocyte of the chick embryo. Differentiation. 1990 Jun;43(3):175–182. doi: 10.1111/j.1432-0436.1990.tb00444.x. [DOI] [PubMed] [Google Scholar]
- Hermos J. A., Mathan M., Trier J. S. DNA synthesis and proliferation by villous epithelial cells in fetal rats. J Cell Biol. 1971 Jul;50(1):255–258. doi: 10.1083/jcb.50.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson B. W., Grund C., Schmid E., Bürki K., Franke W. W., Illmensee K. Formation of cytoskeletal elements during mouse embryogenesis. Intermediate filaments of the cytokeratin type and desmosomes in preimplantation embryos. Differentiation. 1980;17(3):161–179. doi: 10.1111/j.1432-0436.1980.tb01093.x. [DOI] [PubMed] [Google Scholar]
- Kessler M., Acuto O., Storelli C., Murer H., Müller M., Semenza G. A modified procedure for the rapid preparation of efficiently transporting vesicles from small intestinal brush border membranes. Their use in investigating some properties of D-glucose and choline transport systems. Biochim Biophys Acta. 1978 Jan 4;506(1):136–154. doi: 10.1016/0005-2736(78)90440-6. [DOI] [PubMed] [Google Scholar]
- Knapp A. C., Franke W. W. Spontaneous losses of control of cytokeratin gene expression in transformed, non-epithelial human cells occurring at different levels of regulation. Cell. 1989 Oct 6;59(1):67–79. doi: 10.1016/0092-8674(89)90870-2. [DOI] [PubMed] [Google Scholar]
- Kulesh D. A., Ceceña G., Darmon Y. M., Vasseur M., Oshima R. G. Posttranslational regulation of keratins: degradation of mouse and human keratins 18 and 8. Mol Cell Biol. 1989 Apr;9(4):1553–1565. doi: 10.1128/mcb.9.4.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulesh D. A., Oshima R. G. Cloning of the human keratin 18 gene and its expression in nonepithelial mouse cells. Mol Cell Biol. 1988 Apr;8(4):1540–1550. doi: 10.1128/mcb.8.4.1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X., Lane E. B. Retrovirus-mediated transgenic keratin expression in cultured fibroblasts: specific domain functions in keratin stabilization and filament formation. Cell. 1990 Aug 24;62(4):681–696. doi: 10.1016/0092-8674(90)90114-t. [DOI] [PubMed] [Google Scholar]
- Mathan M., Moxey P. C., Trier J. S. Morphogenesis of fetal rat duodenal villi. Am J Anat. 1976 May;146(1):73–92. doi: 10.1002/aja.1001460104. [DOI] [PubMed] [Google Scholar]
- Maunoury R., Robine S., Pringault E., Huet C., Guénet J. L., Gaillard J. A., Louvard D. Villin expression in the visceral endoderm and in the gut anlage during early mouse embryogenesis. EMBO J. 1988 Nov;7(11):3321–3329. doi: 10.1002/j.1460-2075.1988.tb03203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Van Keuren M. L. Gel protein stains: silver stain. Methods Enzymol. 1984;104:441–447. doi: 10.1016/s0076-6879(84)04111-2. [DOI] [PubMed] [Google Scholar]
- Moll R., Franke W. W., Schiller D. L., Geiger B., Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982 Nov;31(1):11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Petell J. K., Quaroni A., Hong W. J., Hixson D. C., Amarri S., Reif S., Bujanover Y. Alteration in the regulation of plasma membrane glycoproteins of the hepatocyte during ontogeny. Exp Cell Res. 1990 Apr;187(2):299–308. doi: 10.1016/0014-4827(90)90095-r. [DOI] [PubMed] [Google Scholar]
- Quaroni A., Calnek D., Quaroni E., Chandler J. S. Keratin expression in rat intestinal crypt and villus cells. Analysis with a panel of monoclonal antibodies. J Biol Chem. 1991 Jun 25;266(18):11923–11931. [PubMed] [Google Scholar]
- Quaroni A. Crypt cell development in newborn rat small intestine. J Cell Biol. 1985 May;100(5):1601–1610. doi: 10.1083/jcb.100.5.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaroni A. Fetal characteristics of small intestinal crypt cells. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1723–1727. doi: 10.1073/pnas.83.6.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaroni A., Isselbacher K. J. Study of intestinal cell differentiation with monoclonal antibodies to intestinal cell surface components. Dev Biol. 1985 Oct;111(2):267–279. doi: 10.1016/0012-1606(85)90482-8. [DOI] [PubMed] [Google Scholar]
- Quaroni A. Pre- and postnatal development of differentiated functions in rat intestinal epithelial cells. Dev Biol. 1985 Oct;111(2):280–292. doi: 10.1016/0012-1606(85)90483-x. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rochette-Egly C., Haffen K. Developmental pattern of calmodulin-binding proteins in rat jejunal epithelial cells. Differentiation. 1987;35(3):219–227. doi: 10.1111/j.1432-0436.1987.tb00172.x. [DOI] [PubMed] [Google Scholar]
- Shibayama T., Carboni J. M., Mooseker M. S. Assembly of the intestinal brush border: appearance and redistribution of microvillar core proteins in developing chick enterocytes. J Cell Biol. 1987 Jul;105(1):335–344. doi: 10.1083/jcb.105.1.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer P. A., Trevor K., Oshima R. G. Molecular cloning and characterization of the Endo B cytokeratin expressed in preimplantation mouse embryos. J Biol Chem. 1986 Jan 15;261(2):538–547. [PubMed] [Google Scholar]
- Stasiak P. C., Purkis P. E., Leigh I. M., Lane E. B. Keratin 19: predicted amino acid sequence and broad tissue distribution suggest it evolved from keratinocyte keratins. J Invest Dermatol. 1989 May;92(5):707–716. doi: 10.1111/1523-1747.ep12721500. [DOI] [PubMed] [Google Scholar]
- Stidwill R. P., Wysolmerski T., Burgess D. R. The brush border cytoskeleton is not static: in vivo turnover of proteins. J Cell Biol. 1984 Feb;98(2):641–645. doi: 10.1083/jcb.98.2.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T. T., Tseng S. C., Huang A. J., Cooper D., Schermer A., Lynch M. H., Weiss R., Eichner R. Monoclonal antibody studies of mammalian epithelial keratins: a review. Ann N Y Acad Sci. 1985;455:307–329. doi: 10.1111/j.1749-6632.1985.tb50419.x. [DOI] [PubMed] [Google Scholar]
- Takemura R., Masaki T., Hirokawa N. Developmental organization of the intestinal brush-border cytoskeleton. Cell Motil Cytoskeleton. 1988;9(4):299–311. doi: 10.1002/cm.970090403. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]