Figure 1.
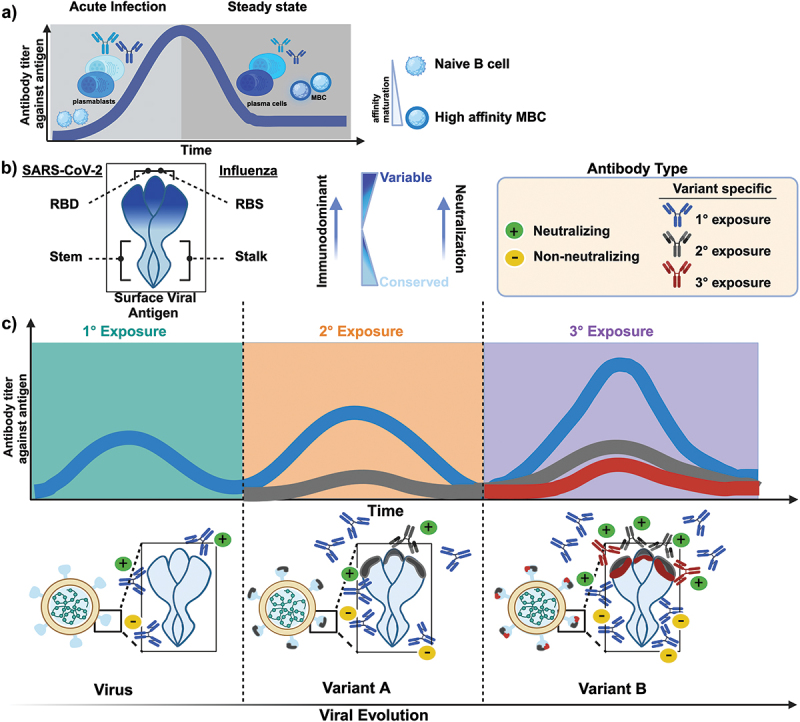
The dynamics of antibody responses following primary and subsequent infections with antigenically distant viruses. a) schematic illustration depicts the dynamics of the humoral response following infection or vaccination. Initially, after the first exposure, a portion of activated B cells differentiate into plasmablasts, which provide an immediate but short-lived source of low-affinity antibodies to limit acute infections. As the humoral response progresses, more specialized responses generate long-lived plasma cells and memory B cells (MBC) which persist at steady state. These cells will rapidly proliferate upon a reencounter with the antigen and become the dominant source of antibodies for subsequent exposures. b) schematic illustration of SARS-CoV-2 and influenza surface viral proteins highlighting the receptor-binding domain (RBD) and/or the receptor-binding site (RBS) as well as the stem and stalk domain, respectively. The gradient represents the more variable, immunodominant domain (dark blue) relative to the more conserved domain (light blue) of the surface viral protein. The RBD/RBS contains numerous immunodominant epitopes and is an important neutralization target given its role in cell-mediated entry. c) the kinetics of antibody responses following primary and subsequent infections with antigenically distant viruses are depicted. The induced humoral response may compromise both recall and de novo responses, depending on the antigenic similarity and cross-reactivity between the infecting variant and previously encountered variants. Primary exposure to an antigen elicits a polyclonal antibody response consisting of both neutralizing and non-neutralizing antibodies. Each subsequent exposure to an antigenically distant viral variant significantly boosts antibodies targeting conserved epitopes while also inducing de novo variant-specific neutralizing responses, albeit at much lower magnitudes. Exposure to an antigenically distinct antigen (variant B) alleviates immunological imprinting and promotes de novo responses targeted to variant-specific epitopes.
