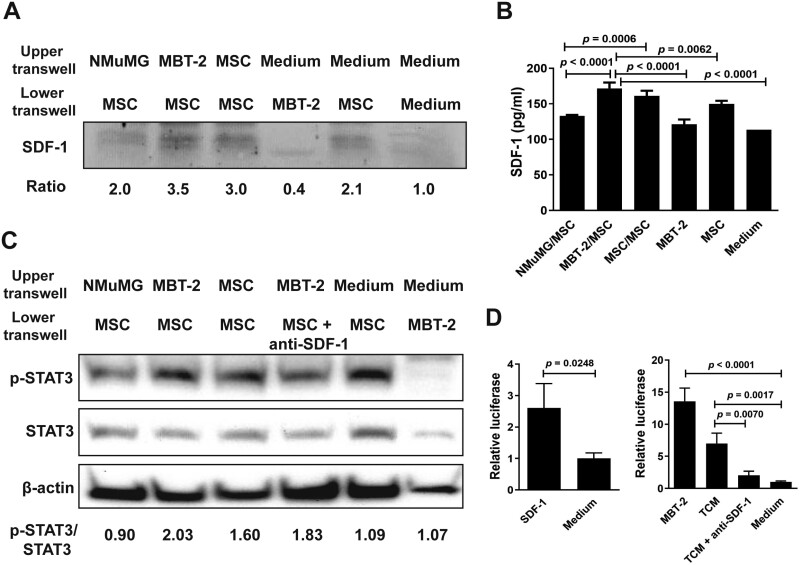
Figure 1.
MSCs exposed to MBT-2 cells or TCM increase SDF-1 secretion, STAT3 activation, and TERT promoter activity. (A-C) Detection of SDF-1, STAT3, and phospho-STAT3 (p-STAT3) in MSCs exposed to MBT-2 cells. NMuMG cells, MBT-2 cells, MSCs, and culture medium were separately added to the upper chamber of the 48-well transwell, whereas MSCs, MBT-2 cells, MSCs plus anti-SDF-1 antibody, and culture medium were separately added to the lower chamber. After culture for 72 h, cells and culture medium in the lower chamber were collected. Levels of SDF-1, p-STAT3, and STAT3 in the cell lysate were detected by immunoblot analysis (A, C). Ratios shown below the blot are the fold increase in the ratio in each lane relative to the ratio obtained from the medium-only control lane analyzed by densitometry (A). SDF-1 content in the culture medium was quantified by ELISA (B). Values shown below the blots are ratios between the intensity of the bands corresponding to p-STAT3 and those corresponding to STAT3 analyzed by densitometry (C). Expression of β-actin served as the loading control. (D) Determination of the TERT promoter activity in MSCs exposed to MBT-2 cells, TCM, or SDF-1. MSCs that had been cotransfected with the luciferase reporter plasmid pGL3-hTERT and pTRELacZ were cocultured with MBT-2 cells, TCM, recombinant SDF-1 protein, TCM plus anti-SDF-1 antibody, or culture medium. Relative luciferase activity was measured by a dual-light luciferase and β-galactosidase reporter gene assay system (Tropix, Bedford, MA) and divided by the β-galactosidase activity to normalize transfection efficiency. (B, D) Values shown are means ± SD (n = 3).