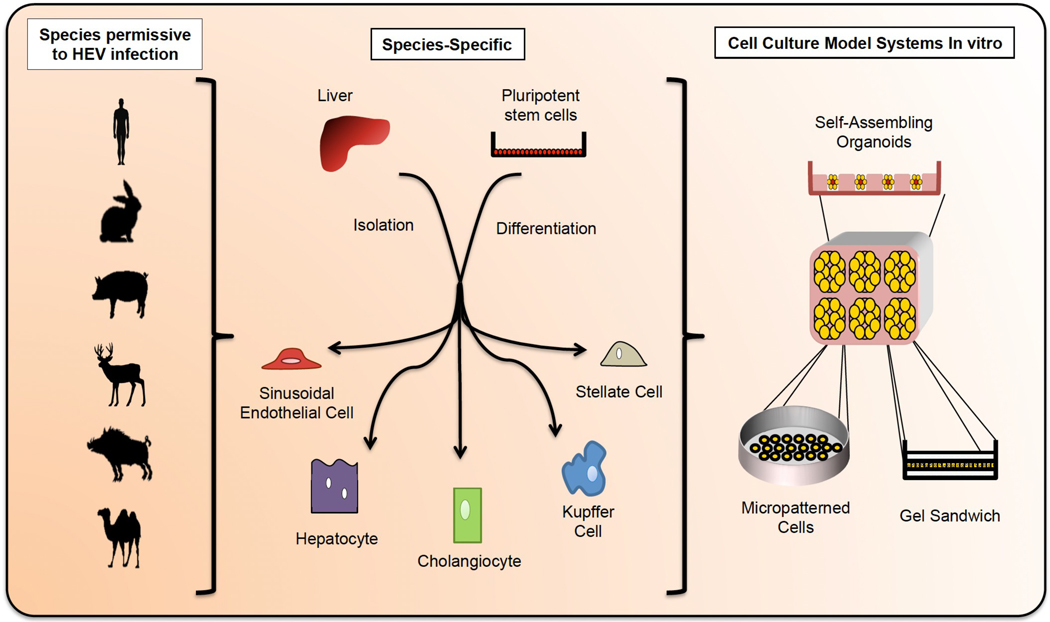
Fig 4. Towards more physiologically relevant 2D and 3D cell culture models for studying HEV.
The species permissive to HEV infection include (but are not limited to) humans, rabbits, swine, deer, wild boar, and camel. In order to better study HEV infection in physiologically relevant in vitro models, it will be desirable to generate co-cultures that recapitulate the complexity of the liver including endothelial, stellate, cholangiocytic, Kupffer, and hepatic cells in the appropriate ratios. These cells can be harvested from primary tissue or differentiated from stem cells, and could be derived from the aforementioned species to explore viral host tropism. Primary cultures have the disadvantage of limited durability; this issue can be overcome by differentiating the various cell types from indefinitely self-renewing stem cells. The latter, however, is technically challenging and requires advances in current hepatic differentiation protocols. Incorporating primary and stem-cell derived tissues into a 3D architecture will also be important to more closely mimic the physiological hepatic environment and preserve cell morphology. The architecture of the liver leads to heterogeneous environmental cues (e.g. nutrients, oxygen, inflammatory factors, etc) reaching individual cells, and 3D cultures can better capture this phenomenon. Furthermore it was previously shown that primary human hepatocyte dedifferentiation can be delayed or prevented in collagen sandwich cultures, by aggregation in spheroids, or in co-culture with non-parenchymal cells.