Abstract
Despite the adenoids are regularly removed in patients with mucopolysaccharidoses (MPS), the underlying tissue and cellular pathologies remain understudied. We characterized an (immuno)histopathologic and ultrastructural phenotype dominated by lysosomal storage changes in a specific subset of adenotonsillar paracortical cells in 8 MPS patients (3 MPS I, 3 MPS II, and 2 MPS IIIA). These abnormal cells were effectively detected by an antibody targeting the lysosomal membrane tetraspanin CD63. Important, CD63+ storage vacuoles in these cells lacked the monocytes/macrophages lysosomal marker CD68. Such a distinct patterning of CD63 and CD68 was not present in a patient with infantile neurovisceral variant of acid sphingomyelinase deficiency. The CD63+ storage pathology was absent in two MPS I patients who either received enzyme-replacement therapy or underwent hematopoietic stem cells transplantation prior the adenoidectomy. Our study demonstrates novel features of lysosomal storage patterning and suggests diagnostic utility of CD63 detection in adenotonsillar lymphoid tissue of MPS patients.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00428-023-03662-y.
Keywords: Mucopolysaccharidoses, Adenoidectomy, CD63, CD68, Enzyme-replacement therapy, Hematopoietic stem cells transplantation
Introduction
Mucopolysaccharidoses (MPS) are a group of eleven rare monogenic disorders caused by a deficiency of one of the lysosomal enzymes involved in glycosaminoglycan (GAG) catabolism. There are seven (I, II, III, IV, VI, VII and IX) distinct MPS phenotypes. All are multisystemic and characterized by progressive lysosomal accumulation (storage) (LS) of partially degraded GAGs and subsequent cellular and tissues dysfunction. Key clinical MPS features include coarse facial features, hepato/splenomegaly, skeletal, joint, cardiac and airway pathologies, hearing impairment, and variable developmental delay. Patients usually appear normal at birth, however, a range of symptoms develops during infancy or early childhood [1]. Some MPS types are amenable to hematopoietic stem cell transplantation (HSCT) and/or recombinant intravenous enzyme-replacement therapy (ERT) [2].
Otorhinolaryngological (ENT) MPS symptoms are frequent and develop early [3, 4]. The most common are chronic/recurrent rhinosinusitis, otitis media (acute and/or with effusion), hearing loss and airway obstruction [3]. Adenotonsillar hypertrophy and recurrent upper airway infections are almost universal and significantly contribute to upper airway obstruction and obstructive sleep apnea syndrome in MPS patients [4]. Although adenoidectomy is frequently performed [3] and histologic evaluation was specifically proposed [5], the structural nature of MPS abnormalities in this lymphoid tissue remains understudied [6–10].
In our report, we present key (immuno)histological- and ultrastructural features of adenotonsillar MPS pathology highlighting the storage changes in CD63+/CD68– lysosomes of specific cells of the lymphoid paracortex.
Materials and methods
Eight MPS patients, one patient with infantile neurovisceral variant of acid sphingomyelinase deficiency (ASMdef, Niemann-Pick disease type A, OMIM 257200), and three age-matched non-MPS/non-LS controls were included in the study (Table 1). Endoscopic adenoidectomy with oral endotracheal intubation was performed under general anesthesia. Mouth gag was inserted to expose the oropharynx. Soft palate was retracted by bilateral rubber catheters passing through the nose. Hypertrophic adenoid tissue was removed using classic adenoid curette under direct transoral endoscopic visualization using a 70° endoscope. Bleeding was controlled by compression with gauze tampons and/or electrocauterization.
Table 1.
Clinical, biochemical, and molecular genetic findings in MPS patients
| Patient #/ Sex/ current age* |
MPS type -mutated gene | Mutations/ residual enzymatic activity |
Age at diagnosis/ first symptoms/ therapy |
ENT manifestations and age at adenoidectomy |
|---|---|---|---|---|
|
1/ F/ 10yrs 7mths |
MPS I – IDUA |
c.[208C > T];[208C > T] p.([Gln70Ter];[Gln70Ter])/ 0.8 nmol/mg/h |
1yr 11mths/ recurrent upper airway infections, cardiomyopathy, developmental delay/ ERT initiated at 2yrs and stopped after HSCT at 2yrs 3mths, enzymatic activity after HSCT 88.8 nmol/mg/h |
mixed hearing loss, tympanostomy tubes placement (4yrs 7mths); second tympanostomy performed together with adenoidectomy (6yrs 7mths); enzymatic activity at the age of adenoidectomy (63.7 nmol/mg/h) |
|
2/ F/ 10yrs 11mths |
MPS I – IDUA (Scheie variant) |
c.[979G > C];[1952del] p.([Ala327Pro];[Gly651AlaextTer?])/ 0.3 nmol/mg/h |
5yrs 5mths/ umbilical hernia, mitral regurgitation with murmur, joint contractures/ ERT initiated at 5yrs 8mths |
tympanostomy tubes placement and adenoidectomy (6yrs 3mths) due to bilateral mixed hearing loss and recurrent middle ear infections |
|
3/ F/ 6yrs 6mths |
MPS I – IDUA |
c.[265C > T];[1205G > A] p.([Arg89Trp];[Trp402Ter])/ 1.3 nmol/mg/h |
4yrs 6mths/ joint contractures, splenomegaly/ ERT initiated at 4yrs 8mths and stopped after HSCT at 5yrs |
adenoidectomy performed (4yrs 7mnths) due to adenoid hypertrophy and upper airway obstruction |
|
4/ F/ 13yrs |
MPS II – IDS (neuronopathic—skewed XCI) |
c.[1403G > A];[ =] p.([Arg468Gln];[ =])/ 0.46 nmol/mg/4h |
3yrs 4mths/ macrosomia, upper airway obstruction/ ERT initiated at 4 yrs and stopped at 8 yrs |
adenoidectomy (2 yrs); hearing impairment diagnosed and hearing aids applied at 4 yrs; Apnoea-Hypopnea index (AHI) – 13; adenotonsillectomy (7yrs 4mths – presented) significantly improved the sleep parameters |
|
5/ M/ 6yrs 8mths |
MPS II – IDS (neuronopathic) |
g.[recombination IDSintr7/IDS2 intr7];[0]/ 0.2 nmol/mg/4h |
1yr 2mths/ inguinal hernia, recurrent airway infections/ ERT initiated at 16 mths and stopped at 2yrs 4mths |
tympanostomy tubes placement and adenoidectomy due to airway obstruction (snoring) were performed at 2yrs 5mths, persistent rhinorrhoea and otitis media with effusion |
|
6/ M/ 7yrs 2mths |
MPS II – IDS (neuronopathic) |
c.[879 + 1G > A];[0]/ RNA mis-splicing < 2,8 μmol/L/h |
2yrs 1mth/ recurrent upper airway infections, developmental delay, hypotonia, umbilical hernia/ ERT initiated at 2yrs 5mths |
adenoidectomy and bilateral tympanostomy (2yrs 8mths) due to recurrent upper airway infections and recurrent middle ear infections, moderate sensorineural hearing loss temporarily improved with hearing aids |
|
7/ F/ 10yrs 9mths |
MPS IIIA – SGSH |
c.[1483 T > C];[1483 T > C] p.([Cys495Arg];[p.Cys495Arg])/ 0.0 nmol/mg/7h |
2yrs/ developmental delay, chronic rhinorrhoea |
adenoidectomy (6 yrs) due to recurrent upper airway infections, persistent rhinorrhoea and snoring |
|
8/ F/ 6yrs |
MPS IIIA – SGSH |
c.[220C > T];[579C > G] p.([Arg74Cys];[Phe193Leu])/ 0.03 nmol/mg/7h |
3yrs 6mths/ developmental delay, recurrent upper airway infections, hepatosplenomegaly/ SRT (Genistein) initiated at 3yrs 8mths |
adenoidectomy (3yrs 9mths) due to adenoid hypertrophy and recurrent upper airway infections |
|
9/ M/ 10yrs 11mths |
ASMdef – SMPD1 |
c.[880C > A];[880C > A] p.([Gln294Lys];[Gln294Lys])/ 0.17 nmol/mg/h |
3yrs 2mths/ hepatosplenomegaly |
adenoidectomy (6yrs 5mths) due to symptoms of upper airway obstruction (snoring, apnoea) and persistent rhinorrhoea |
Mutated genes (reference sequences, control values of enzymatic activity in leukocytes): MPS I – IDUA (NM_000203.5, 18.1–57.5 nmol/mg/h), MPS II—IDS (NM_000202.8, 28.1–93.8 nmol/mg/4 h (patients #4 and #5), ≥ 5,6 umol/L/h (patient #6)), MPS IIIA – SGSH (NM_000199.5, 1.4–11.0 nmol/mg/7 h), ASMdef – SMPD1 (NM_000543.5, 2.25–10.8 nmol/mg/h)
*age on July 31, 2023; ERT—enzyme replacement therapy, HSCT—hematopoietic stem cells transplantation, SRT—substrate reduction therapy, XCI – X-chromosome inactivation
The non-MPS/non-LS controls were 3 patients, who underwent adenoidectomy (aged 4yrs 1mth, 1 yr 9 mths, and 8yrs 4mths) because of a combination of upper airway obstruction symptoms (snoring, rhinolalia clausa, and obstructive sleep apnea syndrome) and recurrent upper airway infections (rhinosinusitis)
Tissues from all individuals (8 MPS, 1 ASMdef, and 3 non-MPS/non-LS controls) were processed for standard light microscopy (HE, PAS, diastase PAS, and Masson´s trichrome), immunohistochemistry (IHC) and immunofluorescence (IF) as described before [11]. Table S1 lists the primary and secondary antibodies (Ab). Confocal imaging and image restoration of the multi-IF stained sections was performed using the Leica SP8X microscope and Huygens Professional Software (SVI, Hilversum, The Netherlands) [12]. Ultrastructural analyses used JEOL 1200 electron microscope (JEOL, Tokyo, Japan) [11]. The study was approved by the authors´ institutional ethical committee (decision no. 2051/18S-IV).
Results
Tissue samples from all tested individuals (including non-MPS/non-LS controls) shared similar nonspecific histopathological inflammatory changes corresponding to findings commonly associated with the clinical diagnosis of adenotonsillar hypertrophy. Lymphoid tissues were covered by locally eroded pseudostratified ciliary respiratory and/or squamous epithelium. Surface accumulation of neutrophil leukocytes and formation of micro-abscesses was seen occasionally. Lymphoid germinal centers were hyperplastic and with abundant vacuolated histiocytes/macrophages. Important, however, MPS patients #3 to #8 showed a population of PAS detectable vacuolated stellate/arachnoid cells in the subepithelial and lymphoid paracortical areas.
Tissue distribution of CD3 positive (+) and CD20+ lymphocytes was usual in all patients (#1–#9, Fig. S1a, b) and control samples (not shown). The vacuolated paracortical cells seen in patients #3–#8 were CD68 negative (–) but strongly CD63+ (Fig. 1a–d; Fig. S1c–f). Other markers (CD3, CD20, CD45RO, CD31, S-100, CD1a, Langerin, desmin, vimentin, and smooth muscle actin) were negative in these vacuolated paracortical cells (Fig. S2).
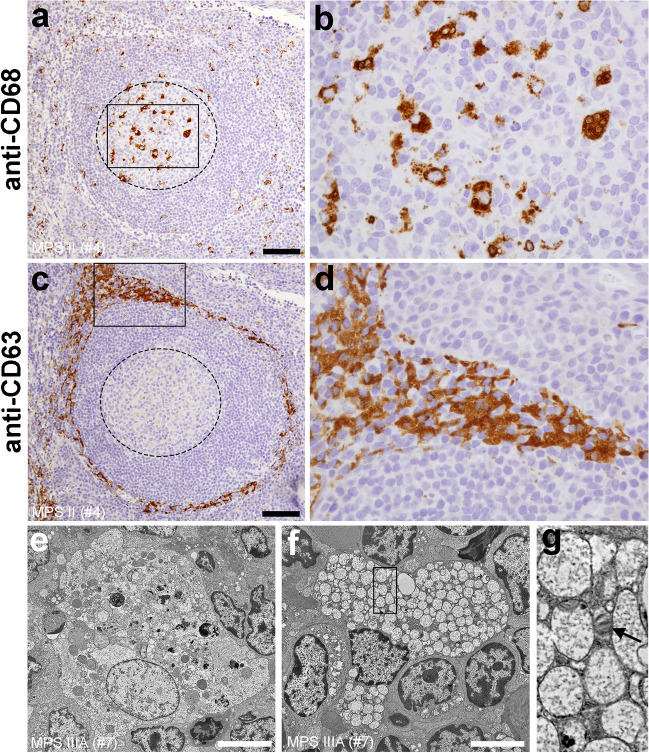
Fig. 1.
Key features of adenotonsillar (immuno)histological and ultrastructural pathology in MPS patients. (a, b) CD68+ macrophages predominate in follicular germinal centers (outlined by dashed line). Image in b corresponds to the rectangle in a; (c, d) vacuolated CD63+ cells populate paracortex. Image in d corresponds to the rectangle in c. a–d images originate from serial histological sections; (e) cytoplasm of follicular germinal center macrophages contains pleoimorphic vacuoles; (f) paracortical cells contain partly cleared membrane-bound storage vacuoles with finely granular contents. (g) occasional zebra bodies can be also detected (black arrow). Image corresponds to the area outlined by the rectangle in f. (a–d) MPS II (patient #4); (e–g) MPS IIIA (patient #7) scale bars = 100 µm (a, c); 2 µm (e, f)
Ultrastructural analyses in all samples showed germinal center macrophages that contained pleomorphic membrane bound vacuoles that lacked the typical structural MPS lysosomal storage properties (Fig. 1e; Fig. S1g). A complex meshwork of (residually) stellate-shaped cells with expanded vacuolated cytoplasm was detected in the paracortical areas (Fig. 1f; Fig. S1h, i) in patients #3–#8. These single membrane-bound vacuoles had typical MPS storage morphology. Majority of them were lucent with fine granular contents. Zebra bodies were seen occasionally (Fig. 1g).
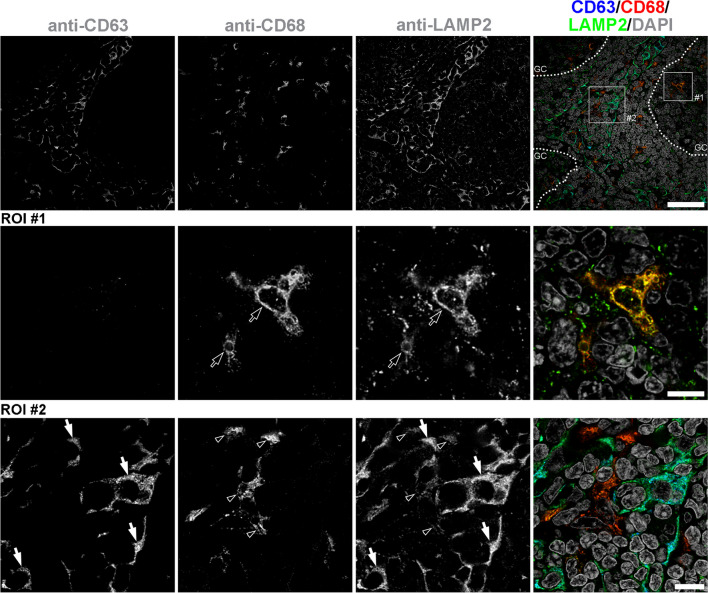
Lysosomal nature of these (CD63+/CD68–) storage vacuoles was confirmed by triple co-labelling with the lysosomal-associated membrane protein 2 (LAMP2) (Fig. 2).
Fig. 2.
Distribution of CD63+ and CD68+ lysosomes in adenotonsillar tissue of MPS patients. Tissue sections were triple-stained with anti-CD63, anti-CD68, and anti-LAMP2 primary antibodies and detected by fluorescently labeled species-specific secondary antibodies (Tab. S1). Dotted lines delineate germinal centers (GC). Region of interest #1 (ROI #1) localizes to GC. ROI #2 localizes to paracortex. GC macrophages (ROI #1, empty arrows) as well as macrophages of the paracortex (ROI #2, empty arrowheads) contain CD68+ lysosomes (LAMP2+) that are CD63–. Specific population of paracortical cells (ROI #2, four highlighted by white arrows) contains excess of CD63+ storage-laden LAMP2+ lysosomes. Representative images are shown (patient #7 – MPS IIIA). Compare to Fig. 1, S1, and S7. Scale bars = 40µm (top row); 10 µm (middle row), 10 µm (bottom row)
Findings (Fig. S3) in patient #1 (MPS I treated by HSCT 4 years and 4 months prior adenoidectomy) and patient #2 (milder MPS I (Scheie) variant treated by ERT for 7 months prior adenoidectomy) (Table 1) were comparable to tissues of non-MPS/non-LS control adenotonsillar tissues (Fig. S4).
CD63 staining was variable in other lysosomal storage affected cells in non-adenotonsillar tissues of MPS patients (Fig. S5). The specific separation of CD63 and CD68 lysosomal patterning to different adenotonsillar paracortical cellular populations seen in MPS patients was not that distinct in a patient with infantile neurovisceral ASMdef (Fig. S6 and S7).
Discussion
Information about adenotonsillar pathology in MPS patients is limited. Sub-epithelial aggregation of histiocytoid cellular forms has been identified earlier by standard tissue stains [8]. Our study combining (immuno)histopathological and ultrastructural techniques characterized adenotonsillar lysosomal storage patterns shared between MPS I, MPS II and MPS IIIA. Except the two treated MPS I patients (#1 and #2), the storage was largely limited to a specific population of paracortical cells that expressed the exosomal/endosomal/lysosomal tetraspanin CD63. Important, this specific population of CD63+ cells/lysosomes lacked CD68, which is a well-established marker of macrophage lysosomal membranes. To further characterize this uniquely distributed cellular population, we performed a series of additional immunological stains including dendritic cell markers (CD3, CD20, CD45RO, CD31, desmin, vimentin, and smooth muscle actin, S-100, CD1a and Langerin) [13, 14]. We did not detect any of these in the storage-ladden CD63+ cells. CD63 is known to localize to endosomal-lysosomal membranes. It has, however, been also implicated in exosomal dynamics, MHCII-associated antigen processing and cellular activation via extracellular cytosplasmic membrane exposure. At this point, we can only hypothesize about the nature of the CD63+/CD68– storage affected paracortical adenotonsillar cells in MPS patients and impacts the lysosomal storage has on mucocilliary dynamics, (secondary) microbial colonization, and nonspecific as well as specific mucosa-associated immunity regulation including antigen processing. The hematopoietic origin of these cells can be implied based on the findings in the tissues of patient #1 who underwent HSCT. Given the tissue distribution, morphology, and possible secondary storage-induced expression abnormalities, we suggest that these cells are more likely of macrophage rather than (interdigitating) dendritic nature [13, 14]. In any case, both these cell types have high endocytic turnover which makes them susceptible to develop lysosomal storage. We also stained adenotonsillar tissues from a patient with infantile neurovisceral ASMdef. While slightly more numerous in comparison to non-MPS/non-LS controls, the population of CD63+/CD68– paracortical cells was much less pronounced in the latter patient than in any of the MPS patients. Contrary to MPS patients, ASMdef patient presented with massive storage in CD63+/CD68+ macrophages (Fig. S6 and S7).
Recent study by Pal et al. [10], demonstrated limited effects of ERT on adenotonsillar lysosomal storage patterns, GAG accumulation and remodeling of extracellular matrix in MPS I, IIIA, IVA, and VI patients. CD63 staining allowed us to compare the extent of cellular storage changes between tissues of treated and untreated patients in our cohort. No or minimal CD63+ lysosomal pathology was detected in patient #1 (adenoidectomy was performed ~ 4 years after HSCT) and patient #2 (adenoidectomy was performed ~ 7 months after ERT initiation). On the contrary, MPS II patient #4 [15] was receiving ERT at the time of adenoidectomy and presented with CD63+ storage changes. Similar, MPS II patients #5 and #6, who stopped receiving ERT 1 and 3 months, respectively, prior the adenoidectomy, also showed CD63+ storage. Last, MPS IIIA patient #8 undergoing substrate reduction treatment for 1 month prior the tissue collection presented with CD63+ lysosomal accumulation comparable to an untreated patient #7.
To conclude. Our data demonstrate unique distribution of storage-laden cells in adenotonsillar paracortical areas of MPS patients. The pattern of lysosomal pathology, which can be very effectively visualized by anti-CD63 immunodetection, is likely shared among various types of MPS(s). We believe that the CD63 tissue detection may facilitate MPS tissue diagnostics and help the assessment of effects of MPS therapies at the cellular and tissue levels.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to acknowledge Lenka Kryspinova, Irena Knesplova, and Marie Kolarova for technical assistance. Drs. Pavel Jesina and Stella Mazurova are acknowledged for coordination of tissue sample collection.
Author contribution
LM, MM, and JSi designed the study. LM, HH, JSt, and JSi performed and evaluated morphological studies. LM, MM, KJ, and MJ collected the clinical data and/or performed the surgery. VB facilitated the confocal imaging analyses. LM, MM, and JSi drafted the manuscript and edited the final version of the manuscript. JSi submitted the manuscript. All authors agreed to the submission of the final version of the manuscript.
Funding
This work was supported by the National Institute for Neurological Research funded by the European Union – Next Generation EU (Program EXCELES, ID Project No. LX22NPO5107), Charles University in Prague (UNCE/MED/007, SVV260516 and Cooperatio), and Ministry of Health of the Czech Republic (RVO-VFN 64165/2012).
Data availability
Not applicable.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
The original version of this article was revised: This article was originally published without an Open Access but due to the authors final decision to opt for Open Choice this correction was created.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
8/23/2024
A Correction to this paper has been published: 10.1007/s00428-024-03902-9
References
- 1.Muenzer J (2011) Overview of the mucopolysaccharidoses. Rheumatology (Oxford) 50(Suppl 5):v4-12. 10.1093/rheumatology/ker394 10.1093/rheumatology/ker394 [DOI] [PubMed] [Google Scholar]
- 2.Taylor M, Khan S, Stapleton M, Wang J, Chen J, Wynn R, Yabe H, Chinen Y, Boelens JJ, Mason RW, Kubaski F, Horovitz DDG, Barth AL, Serafini M, Bernardo ME, Kobayashi H, Orii KE, Suzuki Y, Orii T, Tomatsu S (2019) Hematopoietic Stem Cell Transplantation for Mucopolysaccharidoses: Past, Present, and Future. Biol Blood Marrow Transplant 25:e226–e246. 10.1016/j.bbmt.2019.02.012 10.1016/j.bbmt.2019.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murgasova L, Jurovcik M, Jesina P, Malinova V, Bloomfield M, Zeman J, Magner M (2020) Otorhinolaryngological manifestations in 61 patients with mucopolysaccharidosis. Int J Pediatr Otorhinolaryngol 135:110137. 10.1016/j.ijporl.2020.110137 10.1016/j.ijporl.2020.110137 [DOI] [PubMed] [Google Scholar]
- 4.Simmons MA, Bruce IA, Penney S, Wraith E, Rothera MP (2005) Otorhinolaryngological manifestations of the mucopolysaccharidoses. Int J Pediatr Otorhinolaryngol 69:589–595. 10.1016/j.ijporl.2005.01.017 10.1016/j.ijporl.2005.01.017 [DOI] [PubMed] [Google Scholar]
- 5.Ahmad Z, Kruger K, Lautermann J, Lippert B, Tenenbaum T, Tigges M, Tisch M (2023) Adenoid hypertrophy-diagnosis and treatment: the new S2k guideline. HNO. 10.1007/s00106-023-01299-6 10.1007/s00106-023-01299-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fujitani T, Kimura A, Inoue K, Okada S (1985) Pathological and biochemical study in the adenoid of mucopolysaccharidosis II. Int J Pediatr Otorhinolaryngol 10:205–212. 10.1016/s0165-5876(85)80066-5 10.1016/s0165-5876(85)80066-5 [DOI] [PubMed] [Google Scholar]
- 7.Gonuldas B, Yilmaz T, Sivri HS, Gucer KS, Kilinc K, Genc GA, Kilic M, Coskun T (2014) Mucopolysaccharidosis: Otolaryngologic findings, obstructive sleep apnea and accumulation of glucosaminoglycans in lymphatic tissue of the upper airway. Int J Pediatr Otorhinolaryngol 78:944–949. 10.1016/j.ijporl.2014.03.021 10.1016/j.ijporl.2014.03.021 [DOI] [PubMed] [Google Scholar]
- 8.Keilmann A, Lassig AK, Pollak-Hainz A, Mann WJ, Beck M, Hainz M (2015) Adenoids of patients with mucopolysaccharidoses demonstrate typical alterations. Int J Pediatr Otorhinolaryngol 79:115–118. 10.1016/j.ijporl.2014.11.014 10.1016/j.ijporl.2014.11.014 [DOI] [PubMed] [Google Scholar]
- 9.Nayak DR, Balakrishnan R, Adolph S (1998) Endoscopic adenoidectomy in a case of Scheie syndrome (MPS I S). Int J Pediatr Otorhinolaryngol 44:177–181. 10.1016/s0165-5876(98)00054-8 10.1016/s0165-5876(98)00054-8 [DOI] [PubMed] [Google Scholar]
- 10.Pal AR, Mercer J, Jones SA, Bruce IA, Bigger BW (2018) Substrate accumulation and extracellular matrix remodelling promote persistent upper airway disease in mucopolysaccharidosis patients on enzyme replacement therapy. Plos One 13:e0203216. 10.1371/journal.pone.0203216 10.1371/journal.pone.0203216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dostalova G, Hlubocka Z, Lindner J, Hulkova H, Poupetova H, Vlaskova H, Sikora J, Linhart A, Zeman J, Magner M (2018) Late diagnosis of mucopolysaccharidosis type IVB and successful aortic valve replacement in a 60-year-old female patient. Cardiovasc Pathol 35:52–56. 10.1016/j.carpath.2018.04.001 10.1016/j.carpath.2018.04.001 [DOI] [PubMed] [Google Scholar]
- 12.Bolar NA, Golzio C, Zivna M, Hayot G, Van Hemelrijk C, Schepers D, Vandeweyer G, Hoischen A, Huyghe JR, Raes A, Matthys E, Sys E, Azou M, Gubler MC, Praet M, Van Camp G, McFadden K, Pediaditakis I, Pristoupilova A, Hodanova K, Vyletal P, Hartmannova H, Stranecky V, Hulkova H, Baresova V, Jedlickova I, Sovova J, Hnizda A, Kidd K, Bleyer AJ, Spong RS, Vande Walle J, Mortier G, Brunner H, Van Laer L, Kmoch S, Katsanis N, Loeys BL (2016) Heterozygous loss-of-function SEC61A1 mutations cause autosomal-dominant tubulo-interstitial and glomerulocystic kidney disease with anemia. Am J Hum Genet 99:174–187. 10.1016/j.ajhg.2016.05.028 10.1016/j.ajhg.2016.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collin M, Bigley V (2018) Human dendritic cell subsets: an update. Immunology 154:3–20. 10.1111/imm.12888 10.1111/imm.12888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garces S, Yin CC, Miranda RN, Patel KP, Li S, Xu J, Thakral B, Poppiti RJ, Medina AM, Sriganeshan V, Castellano-Sanchez A, Khoury JD, Garces JC, Medeiros LJ (2020) Clinical, histopathologic, and immunoarchitectural features of dermatopathic lymphadenopathy: an update. Mod Pathol 33:1104–1121. 10.1038/s41379-019-0440-4 10.1038/s41379-019-0440-4 [DOI] [PubMed] [Google Scholar]
- 15.Reboun M, Rybova J, Dobrovolny R, Vcelak J, Veselkova T, Storkanova G, Musalkova D, Hrebicek M, Ledvinova J, Magner M, Zeman J, Peskova K, Dvorakova L (2016) X-Chromosome Inactivation Analysis in Different Cell Types and Induced Pluripotent Stem Cells Elucidates the Disease Mechanism in a Rare Case of Mucopolysaccharidosis Type II in a Female. Folia Biol (Praha) 62:82–89 10.14712/fb2016062020082 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.