Abstract
Background
Mechanical thrombectomy (MT) is playing an increasingly important role in treating deep vein thrombosis (DVT). Although degrees of safety and efficacy have been shown in independent studies, there remains a lack of comparative evidence between MT devices. To address this, we aimed to compare demographics, clinical outcomes, and resource metrics of patients receiving MT for DVT with 3 common devices using a real-world database.
Methods
Patients receiving MT for DVT between January 2018 and March 2022 were identified from the PINC AI Healthcare Database and divided into analysis populations for the AngioJet ZelanteDVT (AJ), the ClotTriever system (CT), and the Indigo system (IN). Rates of in-hospital mortality, resource utilization, and 30-day readmission were compared. Regression modeling was performed to adjust for potential covariates and compare outcomes.
Results
A total of 4455 MT encounters were identified and met inclusion criteria (AJ, 1753; CT, 1344; IN, 1358). In-hospital mortality ranged from 1.0% (CT) to 2.9% (IN), with modeling predicting significantly higher odds for the AJ (odds ratio [OR], 3.42) and IN (OR, 3.38) groups. Similarly, higher rates of resource utilization were predicted in the AJ and IN groups when compared with the reference group (CT). Average costs ranged from $29,549 (CT: SD, $30,705) to $42,705 (IN: SD, $41,114). Thirty-day readmissions ranged from 10.0% (AJ) to 14.6% (IN), while modeling predicted significantly greater odds for the IN group (OR, 1.47).
Conclusions
These results suggest that all MT interventions may be unequal in terms of outcomes and resources, with the CT device associated with lower in-hospital mortality and resource burden.
Keywords: deep vein thrombosis, intervention, lower extremity, real-world data, venous thrombosis
Introduction
Deep vein thrombosis (DVT) is a common and serious disease with 50-80 new diagnoses per 100,000 annually in the United States and can lead to potentially life threatening or debilitating complications when improperly managed.1 Despite the magnitude of this problem, consensus regarding the optimal treatment for DVT is lacking. DVT has traditionally been treated with anticoagulation (AC) therapy. However, although ACs are effective at preventing new thrombus formation, they have limited efficacy in the management of existing thrombus. Evidence shows that up to 50% of patients treated with AC alone develop postthrombotic syndrome, a chronic and debilitating condition.2,3 Interventional techniques aim to provide more immediate symptom relief and reduce long-term complications by dissolving (catheter-directed thrombolysis) or disrupting and extracting thrombus (mechanical thrombectomy [MT]).
The recent emergence of MT as DVT therapy and the subsequent lack of comparative evidence evaluating superiority to the standard of care (AC therapy) has resulted in limited clinical practice guideline recommendations. Three of the most used MT devices for DVT include the AngioJet ZelanteDVT (Boston Scientific), the ClotTriever System (Inari Medical), and the Indigo System (Penumbra). Although modern multicenter registries3, 4, 5, 6, 7, 8, 9 and several single-center experiences10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27 have reported the safety and efficacy of these MT devices, they vary significantly in study design, patient population, collected outcomes, and observed risks. As a result, the evidence on MT for DVT is heterogenous and frequently device specific, which makes evaluation of the differences in device usage and outcomes challenging. Furthermore, many of these studies are small and not necessarily reflective of real-world practice. To address this knowledge gap, we used a large, nationally representative, real-world database to compare outcomes in patients with DVT receiving MT with the AngioJet (AJ), ClotTriever (CT), and Indigo (IN) devices.
Materials and methods
Data source
The PINC AI Healthcare Database (PHD, formerly known as the Premier Healthcare Database; Premier) is a large, US hospital–based, service-level, all-payor database that contains more than 135 million inpatient visits since 2012, representing approximately 25% of annual inpatient admissions in the United States.28 At the time of this study, more than 1164 hospitals contributed data to the PHD, primarily from geographically diverse, nonprofit, nongovernmental community and teaching hospitals and health systems from both rural and urban areas in the United States. Data in the PHD are extracted from standard hospital discharge billing files and include patient demographics and disease characteristics, patient disposition and discharge status, diagnoses codes from admission through discharge, and details of billed services including costs at the departmental level related to medication, medical devices, laboratory tests, and diagnostic and therapeutic services. The data contained in the PHD are statistically deidentified, HIPAA-compliant, and considered exempt from requiring institutional review board approval.
Study population
The analysis population was derived from all available hospital encounters reported in the PHD between January 1, 2018, and March 31, 2022, which met the study inclusion criteria. Encounters were eligible for inclusion if the patient was 18 years or older, the inpatient admission encounter had an International Classification of Diseases (ICD)-10-CM (clinical modification) diagnosis code for acute proximal lower extremity DVT or DVT involving the inferior vena cava (IVC) in any diagnosis position, and the encounter had a documented ICD-10-PCS (Procedure Coding System) code or a documented billed device or service for DVT MT. Encounters were excluded if the diagnosis included pulmonary embolism diagnosis codes, an identifiable device was used that differed from the analysis groups in this study, or the encounter included 2 or more index procedures on the same encounter day.
The PHD contains ICD-10 diagnosis and procedure codes with detailed billing information from a standardized chargemaster record file, an administrative record of billable procedures, equipment fees, supplies, devices, and drugs, among other items during the hospital encounter. To classify DVT interventions from the PHD, a combination of ICD-10 diagnosis, procedure codes, and billing information was used to identify named devices and then matched to 1 of the 3 device groups: the AJ, CT, or IN.
Device descriptions
The AJ device comprised a control unit and catheter. The control unit creates a high pressure, pulsatile jet of saline that exits the catheter tip through multiple retrograde-facing channels. This process creates a localized low-pressure zone and vacuum to accomplish maceration and removal of thrombus through in-flow windows in the catheter. Thrombolytic agents may be administered through the catheter.
The CT device consists of a catheter and funneled sheath. The catheter has an expandable nitinol collection bag and coring element. During treatment, the catheter is advanced beyond the target thrombus, and the collection bag and coring element are expanded. The catheter is then pulled back to the sheath, mechanically dislodging and enveloping the target thrombus. The collection bag is collapsed and retracted through the funneled sheath.
The IN device consists of a control unit and catheter. The control unit maintains a vacuum within the aspiration catheter with a continuous suction to remove thrombus. A wire separator is available for maceration. The IN device is unable to return aspirated blood.
Reported variables and outcomes
Reported baseline encounter and demographic characteristics included age, sex, race and ethnicity, and insurance status. In addition, reported hospital characteristics included geographic region, size, teaching status, and urban-rural classification. Details of thrombus location included presence of thrombus in the IVC, iliac vein, or femoral vein. Documented medical history and comorbidities included a history of venous thromboembolism, status of COVID-19, liver disease, renal disease, malignancy, chronic DVT, and presence of sepsis, DVT, and bleeding risk at admission.
Observed safety outcomes included all-cause in-hospital mortality and all-cause 30-day inpatient readmission. Resource utilization was captured from intraprocedural thrombolytics use, postprocedural blood transfusion, postprocedural hospital stay, postprocedural intensive care unit (ICU) stay, and total hospital encounter costs and charges.
Total hospital encounter costs and charges during the analysis period were determined from the PHD using the standard charge master record file, which details the price and days of services ordered, administered, and billed. Postprocedural transfusions were determined by a documented charge for blood products following the index procedure encounter day. Postprocedural hospital and ICU length of stay (LOS) was defined as the number of encounter days with a documented charge for general or ICU room and board after the index procedure day, respectively.
Statistical analysis
This analysis presents encounter-level clinical outcomes comparing 3 MT groups. Descriptive statistics for all independent variables were reported per MT group using mean and SD for continuous variables and counts and frequency for categorical variables.
Regression modeling was used to examine the relationship between device groups and clinical outcomes while adjusting for potential covariates. For all models, the MT device group with the lowest observed mortality was used as the reference group. Covariates included in the regression model were age, sepsis present on admission, COVID-19 status, mild liver disease, moderate or severe liver disease, renal disease, malignancy, DVT present on admission, a history of venous thromboembolism, and bleeding risk present on admission. Covariates were selected following consultation with physician experts in the field of DVT and authors (D.D. and D.M.).29, 30, 31, 32, 33 A list of ICD-10 codes defining these covariates is included in Supplemental Table 1. For categorical outcomes, multivariable logistic regression was used to estimate odds ratio (OR) and 95% CI for in-hospital mortality, 30-day readmission, postprocedural blood transfusions, and postprocedural ICU stay. For continuous outcomes, multivariable linear regression was used to estimate β coefficients for postprocedural hospital LOS and postprocedural ICU LOS. To adjust for right-skewness, both continuous outcomes of postprocedural hospital LOS (if >20 days) and ICU LOS (if >10 days) were truncated to remove outliers. No imputation was performed to correct for missingness.
Data preparation was performed using Python 3.9.12 (Python Software Foundation), and statistical analysis was performed using RStudio 1.4.1717 (RStudio). A 2-tailed P of <.05 was considered statistically significant for all statistical comparisons.
Results
Study population
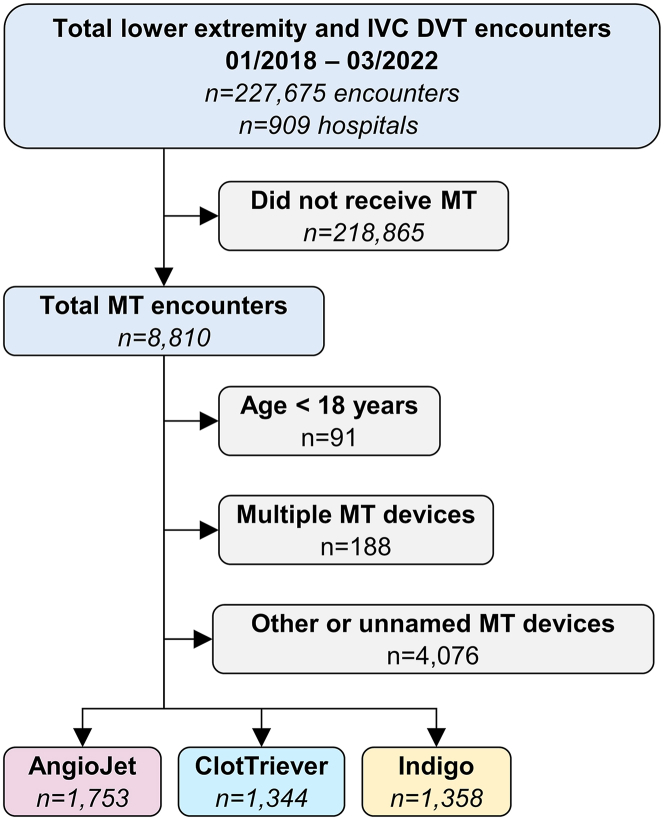
Between January 2018 and March 2022, 227,675 encounters with acute proximal lower extremity and IVC DVT diagnoses from 909 hospitals were included in the study (Figure 1). In total, 218,865 patients did not receive MT in their hospital course, resulting in 8810 identifiable MT encounters. Of those, 91 patients were excluded for age younger than 18 years, 77 were excluded for receiving MT with other identified devices not included in this study, 188 were excluded for receiving treatment with multiple MT devices on the index day of the encounter, and 3999 were excluded for receiving MT with unidentifiable devices. Of the remaining encounters, 1753 (39.3%), 1344 (30.2%), and 1358 (30.5%) were treated with AJ, CT, and IN, respectively. Baseline demographics, thrombus location, comorbidities, and hospital characteristics for the 3 groups are summarized in Table 1.
Figure 1.
Mechanical thrombectomy subject identification from PINC AI Healthcare Database. The distillation of subjects into named device categories used for this analysis.
Table 1.
Demographics, comorbidities, and hospital characteristics.
| AngioJet | ClotTriever | Indigo | |
|---|---|---|---|
| Total encounters, n | 1753 | 1344 | 1358 |
| Demographics | |||
| Age, y | 56.0 ± 16.4 | 60.6 ± 16.8 | 58.7 ± 16.4 |
| Female | 983 (56.1) | 763 (56.8) | 710 (52.3) |
| Race/ethnicity | |||
| White | 1231 (70.2) | 1007 (74.9) | 995 (73.3) |
| Black | 274 (15.6) | 237 (17.6) | 228 (16.8) |
| Hispanic | 150 (8.6) | 106 (7.9) | 159 (11.7) |
| Asian | 16 (0.9) | 6 (0.4) | 16 (1.2) |
| Other | 197 (11.2) | 58 (4.3) | 91 (6.7) |
| Unknown | 35 (2.0) | 36 (2.7) | 25 (2.1) |
| Insurance coverage | |||
| Medicare | 705 (40.2) | 684 (50.9) | 624 (46.0) |
| Managed Care/Commercial | 651 (37.1) | 367 (27.3) | 390 (28.7) |
| Medicaid | 252 (14.4) | 181 (13.5) | 224 (16.5) |
| Other | 66 (3.8) | 50 (3.7) | 57 (4.2) |
| Self-pay | 79 (4.5) | 62 (4.6) | 63 (4.6) |
| Thrombus location | |||
| Inferior vena cava | 291 (16.6) | 229 (17.0) | 236 (17.4) |
| Iliac | 1070 (61.0) | 847 (63.0) | 670 (49.3) |
| Femoral | 117 (67.1) | 1035 (77.0) | 929 (68.4) |
| Unspecified | 509 (29.0) | 354 (26.3) | 394 (29.0) |
| Comorbidities | |||
| History of VTE | 537 (30.6) | 306 (22.8) | 412 (30.3) |
| Sepsis, present on admission | 49 (2.8) | 55 (4.1) | 59 (4.3) |
| COVID-19 | 25 (1.4) | 36 (2.7) | 39 (2.9) |
| Liver disease, mild | 72 (4.1) | 60 (4.5) | 69 (5.1) |
| Liver disease, moderate/severe | 7 (0.4) | 10 (0.7) | 6 (0.4) |
| Renal disease | 250 (14.3) | 223 (16.6) | 239 (17.6) |
| Any malignancy | 143 (8.2) | 164 (12.2) | 150 (11.0) |
| Chronic DVT | 244 (13.9) | 136 (10.1) | 184 (13.5) |
| DVT, present on admission | 1694 (96.6) | 1303 (96.9) | 1270 (93.5) |
| Bleeding risk, present on admission | 177 (10.1) | 105 (7.8) | 140 (10.3) |
| Hospital characteristics | |||
| Hospital region | |||
| Midwest | 425 (24.2) | 463 (34.4) | 194 (14.3) |
| Northeast | 200 (11.4) | 76 (5.7) | 121 (8.9) |
| South | 963 (54.9) | 732 (54.5) | 862 (63.5) |
| West | 165 (9.4) | 73 (5.4) | 181 (13.3) |
| Urban (vs rural) | 1617 (92.2) | 1269 (94.4) | 1255 (92.4) |
| Teaching status | 950 (54.2) | 663 (49.2) | 735 (54.1) |
| No. of beds | |||
| <200 | 168 (9.6) | 145 (10.8) | 143 (10.5) |
| 200-500 | 862 (49.2) | 714 (53.1) | 660 (48.6) |
| >500 | 723 (41.2) | 485 (36.1) | 555 (40.9) |
Values are mean ± SD or n (%), unless specified.
Mortality
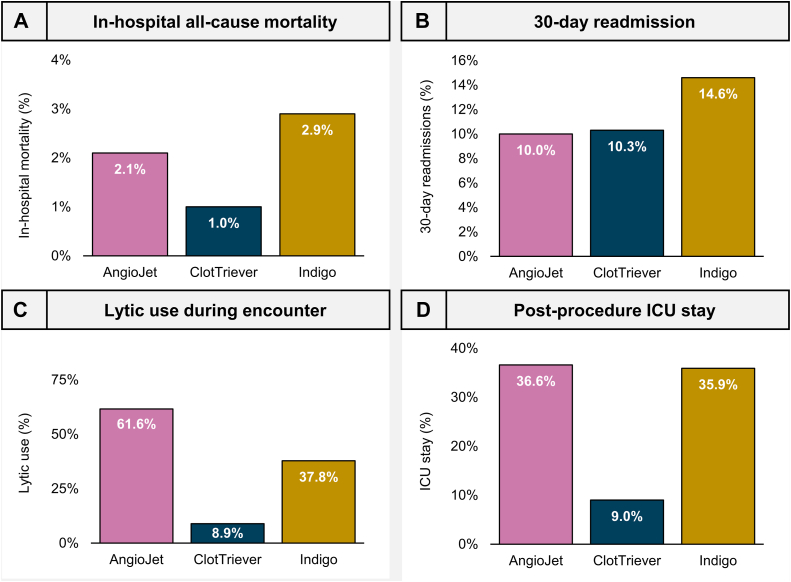
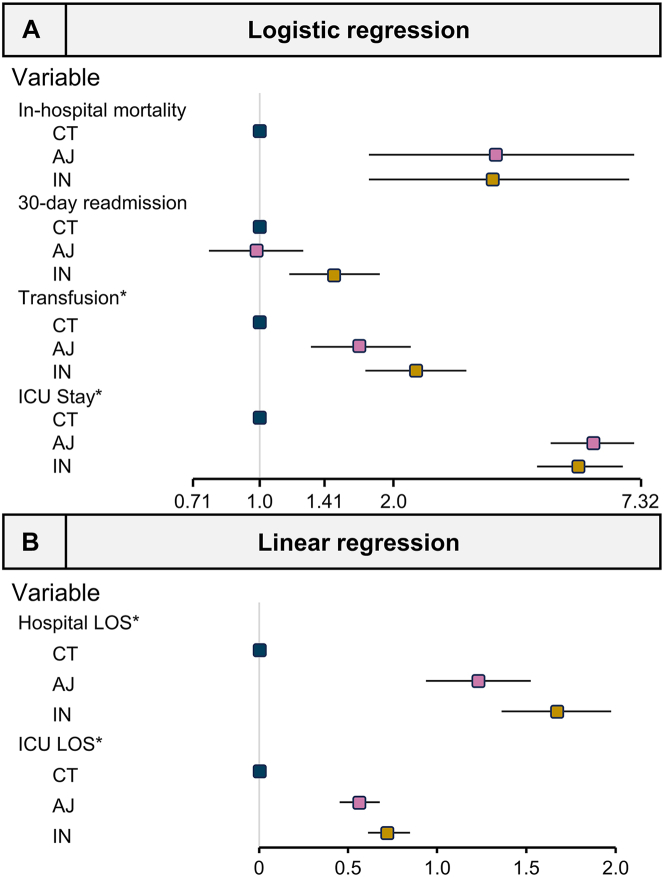
The all-cause in-hospital mortality rate was 2.1% (n = 36), 1.0% (n = 13), and 2.9% (n = 39) for the AJ, CT, and IN groups, respectively (Figure 2A). The CT group was selected as the reference device for regression modeling in this study based on observed in-hospital mortality rates. After adjusting for comorbidities, subjects receiving MT with the AJ (OR, 3.42; P < .001) and the IN (OR, 3.38; P < .001) devices were at significantly higher odds of in-hospital mortality when compared with the CT group (Table 2, Central Illustration). Other variables significantly associated with mortality included age, sepsis present on admission, COVID-19, moderate or severe liver disease, renal disease, malignancy, or DVT present on admission (Table 2).
Figure 2.
Observed outcomes and resource utilization. Observed rates of (A) in-hospital all-cause mortality, (B) all-cause 30-day readmission, (C) patients receiving any adjunctive thrombolytic therapy during the hospital encounter, and (D) patients receiving ICU monitoring postprocedure.
Table 2.
Logistic regression model results for in-hospital mortality, 30-d readmission, and all-cause postprocedural transfusions.
| Predictors | In-hospital mortality (%) |
All-cause 30-d readmission (%) |
Postprocedural transfusion (%) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | P | Odds ratio | 95% CI | P | Odds ratio | 95% CI | P | |
| (Intercept) | 0.00 | 0.00-0.02 | <.001 | 0.20 | 0.11-0.35 | <.001 | 0.12 | 0.07-0.21 | <.001 |
| AngioJet | 3.42 | 1.77-7.04 | <.001 | 0.98 | 0.77-1.25 | .875 | 1.68 | 1.30-2.19 | <.001 |
| Indigo | 3.38 | 1.77-6.85 | <.001 | 1.47 | 1.16-1.86 | .001 | 2.25 | 1.74-2.93 | <.001 |
| Age | 1.02 | 1.00-1.04 | .024 | 0.99 | 0.99-1.00 | .009 | 1.00 | 0.99-1.01 | .817 |
| Sepsis, present on admission | 4.40 | 2.35-7.91 | <.001 | 1.47 | 0.93-2.25 | .085 | 1.85 | 1.23-2.74 | .002 |
| COVID-19 | 9.27 | 4.60-17.84 | <.001 | 1.34 | 0.70-2.39 | .347 | 1.87 | 1.08-3.10 | .019 |
| Liver disease, mild | 1.96 | 0.78-4.20 | .111 | 1.32 | 0.87-1.96 | .178 | 1.10 | 0.71-1.66 | .653 |
| Liver disease, moderate/severe | 9.34 | 1.95-32.47 | .001 | 2.13 | 0.67-5.69 | .157 | 1.23 | 0.34-3.54 | .726 |
| Renal disease | 2.35 | 1.43-3.80 | .001 | 1.62 | 1.27-2.06 | <.001 | 1.45 | 1.13-1.84 | .003 |
| Any malignancy | 2.27 | 1.24-3.97 | .005 | 2.07 | 1.58-2.68 | <.001 | 1.81 | 1.37-2.36 | <.001 |
| DVT, present on admission | 0.25 | 0.14-0.46 | <.001 | 0.65 | 0.44-1.00 | .041 | 0.43 | 0.30-0.62 | <.001 |
| History of VTE | 0.63 | 0.35-1.07 | .100 | 1.18 | 0.96-1.44 | .111 | 1.07 | 0.86-1.31 | .553 |
| Bleeding risk, present on admission | 1.73 | 0.94-3.02 | .066 | 1.80 | 1.36-2.34 | <.001 | 3.56 | 2.79-4.52 | <.001 |
DVT, deep vein thrombosis; MT, mechanical thrombectomy; VTE, venous thromboembolism.
Central Illustration.
Outcomes from regression analysis. (A) Adjusted odds ratios for in-hospital mortality, all-cause 30-day readmission, postprocedural transfusion, and postprocedural ICU stay. (B) Adjusted estimates for postprocedural hospital and ICU LOS. Both panels compare encounters with AJ and IN with CT encounters. ∗Postprocedure. AJ, AngioJet; CT, ClotTriever; IN, Indigo.
The all-cause in-hospital mortality rate for patients with isolated IVC thrombus was 10% (n = 2/20), 0% (n = 0/18), and 0% (n = 0/23) for the AJ, CT, and IN groups, respectively. Similarly, mortality rates for patients with IVC-involved DVT were 2.4% (n = 7/291), 0.9% (n = 2/229), and 0.8% (n = 2/236) for the AJ, CT, and IN groups, respectively.
Readmission
The all-cause 30-day readmission rate was 10.0% (n = 175), 10.3% (n = 138), and 14.6% (n = 198) for the AJ, CT, and IN groups, respectively (Figure 2B). When analyzed with logistic regression modeling and with reference to the CT group, subjects undergoing MT with the IN (OR, 1.47; P = .001) device were at significantly higher odds of requiring 30-day readmission (Table 2, Central Illustration). Other variables significantly associated with 30-day readmission included age, renal disease, malignancy, and bleeding risks.
Thrombolytics use
The rate of adjunctive thrombolytics use was observed to be 61.6% (n = 1080), 8.9% (n = 120), and 37.8% (n = 513) for the AJ, CT, and IN groups, respectively (Figure 2C).
Transfusions
The postprocedural transfusion rate was 11.5% (n = 202), 7.4% (n = 100), and 15.8% (n = 214) for the AJ, CT, and IN groups, respectively. When analyzed with regression modeling and with reference to the CT group, subjects undergoing MT with AJ (OR, 1.68; P < .001) and IN (OR, 2.25; P < .001) devices were at significantly higher odds of requiring transfusion postprocedurally (Table 2, Central Illustration). Other variables significantly associated with postprocedural transfusion included sepsis present on admission, COVID-19, renal disease, malignancy, DVT present on admission, and bleeding risks.
Postprocedural hospital and ICU stay
The average postprocedural hospital LOS was 4.7, 3.5, and 5.8 days for the AJ, CT, and IN groups, respectively. Postprocedural rates of ICU stay were 36.6% (n = 641), 9.0% (n = 121), and 35.9% (n = 487) (Figure 2D) and the average postprocedural ICU LOS was 0.9, 0.2, and 1.2 days, for the AJ, CT, and IN groups, respectively. Logistic regression modeling found that AJ (OR, 5.64; P < .001) and IN (OR, 5.27; P < .001) encounters had significantly greater odds of postprocedural ICU stay than CT encounters (Table 3, Central Illustration). In addition, modeling predicted that patients treated with AJ and IN had a significantly longer postprocedural hospital course (AJ, P < .001; IN, P < .001) and ICU stay (AJ, P < .001; IN, P < .001) when compared with patients treated with CT (Table 3, Central Illustration). Other variables significantly associated with ICU monitoring postprocedural included age, sepsis present on admission, COVID-19, moderate or severe liver disease, renal disease, malignancy, DVT present on admission, and bleeding risks.
Table 3.
Model results for postprocedural ICU stay rate (logistic regression) and postprocedural hospital and ICU LOS (linear regression).
| Predictors | Postprocedural ICU stay (%) |
Postprocedural hospital LOS |
Postprocedural ICU LOS |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | P | Estimates | 95% CI | P | Estimates | 95% CI | P | |
| (Intercept) | 0.18 | 0.12-0.29 | <.001 | 5.55 | 4.77-6.32 | <.001 | 1.71 | 1.42-2.01 | <.001 |
| AngioJet | 5.64 | 4.57-7.02 | <.001 | 1.23 | 0.93-1.52 | <.001 | 0.56 | 0.45-0.67 | <.001 |
| Indigo | 5.27 | 4.24-6.59 | <.001 | 1.67 | 1.36-1.97 | <.001 | 0.72 | 0.61-0.84 | <.001 |
| Age | 0.99 | 0.99-1.00 | .024 | 0.00 | −0.01 to 0.01 | .833 | 0.00 | −0.01 to 0.00 | .817 |
| Sepsis, present on admission | 1.35 | 0.94-1.94 | <.001 | 3.18 | 2.53-3.82 | <.001 | 0.59 | 0.34-0.84 | .002 |
| COVID-19 | 1.47 | 0.93-2.31 | <.001 | 1.30 | 0.50-2.11 | .002 | 0.522 | 0.21-0.83 | .019 |
| Liver disease, mild | 1.21 | 0.87-1.67 | .111 | 1.36 | 0.79-1.93 | <.001 | 0.22 | 0.00-0.44 | .653 |
| Liver disease, moderate/severe | 2.65 | 1.04-6.59 | .001 | 3.39 | 1.73-5.05 | <.001 | 0.93 | 0.29-1.56 | .726 |
| Renal disease | 1.20 | 0.99-1.46 | .001 | 1.57 | 1.24-1.91 | <.001 | 0.33 | 0.20-0.49 | .003 |
| Any malignancy | 0.85 | 0.66-1.08 | .005 | 0.65 | 0.25-1.04 | .001 | −0.06 | −0.21 to 0.09 | <.001 |
| DVT, present on admission | 0.72 | 0.52-1.00 | <.001 | −3.27 | −3.87 to 2.67 | <.001 | −1.42 | −1.65 to 1.19 | <.001 |
| History of VTE | 1.08 | 0.93-1.26 | .100 | 0.51 | 0.24-0.77 | <.001 | −0.05 | −0.15 to 0.05 | .553 |
| Bleeding risk, present on admission | 1.02 | 0.81-1.28 | .066 | 1.98 | 1.58-2.39 | <.001 | 0.34 | 0.18-0.50 | <.001 |
| Preprocedural ICU stay | 2.54 | 2.05-3.15 | <.001 | ||||||
DVT, deep vein thrombosis; MT, mechanical thrombectomy; VTE, venous thromboembolism.
Charges and costs
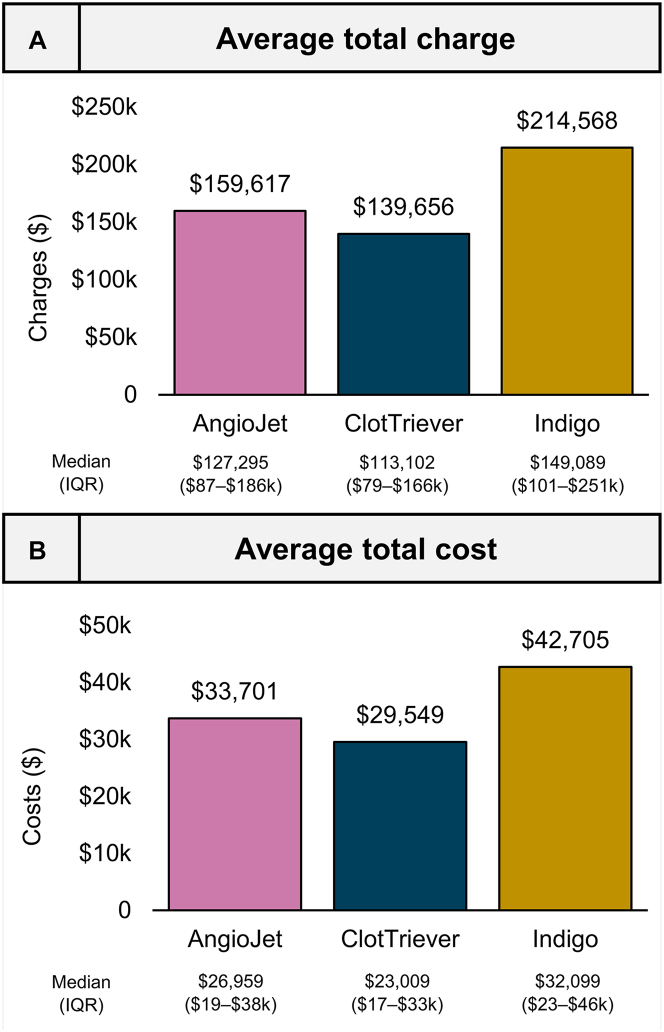
Mean and median total encounter charges and costs are presented in Figure 3. The mean total charge per encounter was $159,617 (SD, $155,547), $139,656 (SD, $110,301), and $214,567 (SD, $227,606) for the AJ, CT, and IN groups. The mean total encounter cost incurred by the hospital per encounter was $33,701 (SD, $32,985), $29,549 (SD, $30,705), and $42,705 (SD, $41,114), respectively.
Figure 3.
Observed charges and costs. (A) Average total charges (graph) and median total charges (tabulated) per device encounter. (B) Average cost incurred by hospital (graph) and median cost incurred (tabulated) per device encounter.
Discussion
This large analysis of patients with acute DVT, who were treated with MT between January 2018 and March 2022, provides significant insights into the real-world practice of DVT management. Our results suggest that there may be differences in clinical outcomes and resource utilization based on the type of MT device used. These findings emphasize that in real-world DVT management there may be a relationship between device selection and outcomes.
Safety outcomes
Observed in-hospital mortality rates for each device group were low (1.0%-2.9%) and appear consistent with pevious studies. For comparison, in-hospital mortality has been reported to be 0.6% for CT9,16 and 30-day mortality has been reported to be up to 4.0% for AJ6, 7, 8 in large prospective cohorts. Similar previous evidence for the IN device was unavailable as contemporary publications are limited to retrospective analysis of small populations.22, 23, 24, 25, 26 Further, logistic-regression modeling in comparison with the lowest observed mortality group (CT) suggested that there were significantly higher odds of in-hospital mortality with AJ or IN treatment (OR, 3.38-3.42). Although the lack of a controlled study population limits definitive conclusions, multiple hypotheses exist for these observed mortality differences. It is possible that differences in device effectiveness can result in residual thrombus, leading to more acute safety events. Alternatively, these relative differences in safety can stem from the differential usage of adjunctive thrombolytics.
All-cause 30-day readmission has been reported at 0%-7.3% in previous studies of CT.4,16 Similar rates are unavailable for the AJ and IN groups. From the PHD, we see elevated readmission rates in real-world practice (10.0%-14.6%). Further, logistic-regression modeling predicted significantly greater 30-day readmission rates associated with the IN group compared with that of the CT group.
Resource utilization
Thrombolytic use is relatively contraindicated in up to 30%-40% of patients with DVT due to an increased risk of bleeding related to comorbidities or medical history.9,16 Meta-analyses of patients with DVT treated with thrombolytics have revealed increased major bleeding rates (4.5%-6.7%) and predict up to a 2.5× increased risk in comparison with treatment with ACs alone.34,35 Rates of adjunctive thrombolytics use in MT procedures have been reported as 67% for AJ,7 0%-0.4% for CT,9,16 and ranging 25%-70% for IN.22,26 Our study observed relatively similar thrombolytic use from the PHD. It is possible that greater thrombolytics use may contribute to higher rates of transfusion, in-hospital mortality, and ICU stay.
Blood transfusions during hospitalization have been previously associated with poorer clinical outcomes.36 For example, in patients having cardiac surgery, postprocedural transfusions have been linked to increased postoperative mortality and morbidity necessitating further hospital and ICU stay, and overall admission-related costs.36 In previous studies, postprocedural transfusion rates have been reported at 2.4% for AJ6 and 0.6% for CT9; however, we observed rates of postprocedural transfusion in this study ranging 7.4%-15.8%. Our findings could be explained by the unrestricted, real-world nature of the analysis population in contrast to previous studies. We also observed that patients treated with CT had lower odds of requiring postprocedural transfusion than other device groups. Although we are unable to determine the cause of this difference, we suspect that the higher rate of thrombolytic use for these groups and the associated possibility of increased bleeding risks may be partially responsible. It is also possible that increased transfusion requirements predicted for the IN group are due to its continuous aspiration mechanism of action, which theoretically can result in high blood loss.
Rates of patients requiring postprocedural ICU stay ranged from 9.0% (CT) to 36.6% (AJ). From the literature, ICU rates have been reported to be 0%-2.2% for the CT9,16 device and 6%-50% for the IN22,24,26 device. Similarly, this analysis predicted longer hospital and ICU stay for the other named device groups when compared with those for the CT device. It is likely that patients receiving more thrombolytics, as seen with the AJ and IN groups in this analysis, require closer observation postprocedural due to the added bleeding risks.
Charges and costs are heavily tied to resource utilization throughout the patient encounter and include additional factors beyond the direct cost of a device (eg, pharmaceutical agents, administered blood products, and postprocedural stay). These findings may in part be explained by the lower observed rate of thrombolytic use, lower predicted transfusion needs, and both observed and predicted lower rates of postprocedural ICU stay for CT encounters. More health economics–based research is needed to explain these findings in detail. Presently, device selection is frequently left to physician preference which may lead to provider-specific disparities in quality and cost.
Study limitations
Although true for any retrospective database analysis, there is potential for selection bias in this study. As an observational study of real-world hospital administrative data, encounter selection was based on administrative coding and billing data and may be subject to human or site-specific variability in collection. Further, diagnostic and therapeutic rationale, imaging and laboratory results, the severity and chronicity of disease burden, and other factors leading to treatment selection are not ascertainable from the PHD. Encounters with concomitant PE or with multiple procedures on the index day were excluded, as the sequence of procedures is unable to be ascertained. The PHD also has the potential to misclassify reported outcomes and is unable to directly evaluate longitudinal outcomes (eg, vein patency or the rate of subsequent PE), details pertaining to treatment compliance (eg, AC regimen), or further details related to mortality (eg, cause of death). Only encounters with conservatively identified MT devices were included in their respective device group. Causation cannot be proven in the observed findings, and comparative results cannot rule out selection bias or unmeasured confounders.
Conclusion
Results from this large real-world database suggest that all MT interventions may not be equal for the treatment of DVT, with certain devices being associated with favorable safety and lower resource burden. Further prospective studies are needed to validate these results and thereby help shape the DVT treatment landscape.
Acknowledgments
Declaration of competing interests
Derek Mittleider is a consultant for Boston Scientific and Inari Medical. C. Michael Gibson received research grant support from Inari Medical, Boston Scientific, and Penumbra and consultant for Inari Medical. David Dexter is a consultant for AngioDynamics, Boston Scientific, Penumbra, and Inari Medical.
Funding sources
This work was not supported by funding agencies in the public, commercial, or not-for-profit sectors.
Ethical statement and patient consent
The reported study has adhered to all relevant ethical guidelines, used only deidentified medical records, and did not require institutional review board approval.
Footnotes
To access the supplementary material accompanying this article, visit the online version of the Journal of the Society for Cardiovascular Angiography & Interventions at 10.1016/j.jscai.2024.102149.
Supplementary material
References
- 1.Baekgaard N. Incidence and location of deep vein thrombosis in the lower extremities: what do we know. Phlebolymphology. 2017;24:97–104. [Google Scholar]
- 2.Kahn S.R., Shrier I., Julian J.A., et al. Determinants and time course of the postthrombotic syndrome after acute deep venous thrombosis. Ann Intern Med. 2008;149(10):698–707. doi: 10.7326/0003-4819-149-10-200811180-00004. [DOI] [PubMed] [Google Scholar]
- 3.Vedantham S., Goldhaber S.Z., Julian J.A., et al. Pharmacomechanical catheter-directed thrombolysis for deep-vein thrombosis. N Engl J Med. 2017;377(23):2240–2252. doi: 10.1056/NEJMoa1615066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dexter D.J., Kado H., Schor J., et al. Interim outcomes of mechanical thrombectomy for deep vein thrombosis from the All-Comer CLOUT Registry. J Vasc Surg Venous Lymphat Disord. 2022;10(4):832–840.e2. doi: 10.1016/j.jvsv.2022.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Maldonado T.S., Dexter D.J., Kado H., et al. Outcomes from the ClotTriever Outcomes Registry show symptom duration may underestimate deep vein thrombus chronicity. J Vasc Surg Venous Lymphat Disord. 2022;10(6):1251–1259. doi: 10.1016/j.jvsv.2022.04.015. [DOI] [PubMed] [Google Scholar]
- 6.Garcia M.J., Lookstein R., Malhotra R., et al. Endovascular management of deep vein thrombosis with rheolytic thrombectomy: final report of the prospective multicenter PEARL (Peripheral use of angiojet rheolytic thrombectomy with a variety of catheter lengths) registry. J Vasc Interv Radiol. 2015;26(6):777–785. doi: 10.1016/j.jvir.2015.01.036. [DOI] [PubMed] [Google Scholar]
- 7.Leung D.A., Blitz L.R., Nelson T., et al. Rheolytic pharmacomechanical thrombectomy for the management of acute limb ischemia: results from the PEARL registry. J Endovasc Ther. 2015;22(4):546–557. doi: 10.1177/1526602815592849. [DOI] [PubMed] [Google Scholar]
- 8.Vedantham S., Salter A., Lancia S., Lewis L., Thukral S., Kahn S.R. Clinical outcomes of a pharmacomechanical catheter-directed venous thrombolysis strategy that included rheolytic thrombectomy in a multicenter randomized trial. J Vasc Interv Radiol. 2021;32(9):1296–1309.e7. doi: 10.1016/j.jvir.2021.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dexter D., Kado H., Shaikh A., et al. Safety and effectiveness of mechanical thrombectomy from the fully enrolled multicenter, prospective CLOUT Registry. J Soc Cardiovasc Angiogr Interv. 2023;2(2) doi: 10.1016/j.jscai.2023.100585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benarroch-Gampel J., Amit P., Aizpuru M., Rajani R., Jordan W., Crawford R. Technical success and short-term outcomes after treatment of lower extremity deep vein thrombosis with the ClotTriever system: a preliminary experience. J Vasc Surg Venous Lymphat Disord. 2020;8(2):174–181. doi: 10.1016/j.jvsv.2019.10.024. [DOI] [PubMed] [Google Scholar]
- 11.Zia S., Juneja A., Chadnick Z., et al. Provisional review and comparison of ClotTreiver mechanical thrombectomy versus catheter directed and other mechanical thrombolysis and thrombectomy modalities for lower extremity deep venous thrombosis. J Vasc Surg. 2020;72(1):E243–E244. [Google Scholar]
- 12.Irshad A., Shah P.S., Jain A., et al. Promising novel technique for percutaneous extraction of chronic lower extremity deep venous thrombosis: clinical experience with the ClotTriever system. J Vasc Surg. 2020;72(1):e60–e61. [Google Scholar]
- 13.Raskin A., Verma A., Brennan T.D. Single-session thrombolysis-free treatment of deep vein thrombosis with a novel mechanical thrombectomy device. J Am Coll Cardiol Case Rep. 2021;3(3):415–420. doi: 10.1016/j.jaccas.2020.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah N.G., Wible B.C., Paulisin J.A., et al. Management of inferior vena cava thrombosis with the FlowTriever and ClotTriever systems. J Vasc Surgery Venous Lymphat Disord. 2021;9(3):615–620. doi: 10.1016/j.jvsv.2020.09.008. [DOI] [PubMed] [Google Scholar]
- 15.Wadhwa V., Malhotra A., Kesselman A. Single-session mechanical thrombectomy of lower extremity deep venous thrombosis using the ClotTriever system: a single-institution experience. Arab J Interv Radiol. 2021;05:71–75. [Google Scholar]
- 16.Jolly M.A., Lockhart M.M., Shah D., et al. Outcomes from a tertiary care center using a catheter thrombectomy system for managing acute iliofemoral deep vein thrombosis. J Vasc Surg Venous Lymphat Disord. 2022;10(5):1044–1050. doi: 10.1016/j.jvsv.2022.04.010. [DOI] [PubMed] [Google Scholar]
- 17.Weissler E.H., Cox M., Commander S., Williams Z. Restoring venous patency with the ClotTriever following deep vein thrombosis. Ann Vasc Surg. 2022;88:268–273. doi: 10.1016/j.avsg.2022.07.031. [DOI] [PubMed] [Google Scholar]
- 18.Morrow K.L., Kim A.H., Plato S.A., et al. Increased risk of renal dysfunction with percutaneous mechanical thrombectomy compared with catheter-directed thrombolysis. J Vasc Surg. 2017;65(5):1460–1466. doi: 10.1016/j.jvs.2016.09.047. [DOI] [PubMed] [Google Scholar]
- 19.Salem K.M., Saadeddin Z., Go C., et al. Risk factors for acute kidney injury after pharmacomechanical thrombolysis for acute deep vein thrombosis. J Vasc Surg Venous Lymphat Disord. 2021;9(4):868–873. doi: 10.1016/j.jvsv.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen Y., Wang X., Jin S., Zhang R., Zhao W., Chen G. Increased risk of acute kidney injury with percutaneous mechanical thrombectomy using AngioJet compared with catheter-directed thrombolysis. J Vasc Surg Venous Lymphat Disord. 2019;7(1):29–37. doi: 10.1016/j.jvsv.2018.06.016. [DOI] [PubMed] [Google Scholar]
- 21.Escobar G., Burks D., Abate M., et al. Risk of acute kidney injury after percutaneous pharmacomechanical thrombectomy using AngioJet in venous and arterial thrombosis. Ann Vasc Surg. 2017;42:238–245. doi: 10.1016/j.avsg.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 22.Saleem T., Fuller R., Raju S. Aspiration mechanical thrombectomy for treatment of acute iliofemoral and central deep venous thrombosis. Ann Vasc Surg Brief Rep Innov. 2021;1(2) [Google Scholar]
- 23.Teter K., Arko F., Muck P., et al. Aspiration thrombectomy for the management of acute deep venous thrombosis in the setting of venous thoracic outlet syndrome. Vascular. 2019;28(2):183–188. doi: 10.1177/1708538119895833. [DOI] [PubMed] [Google Scholar]
- 24.Robertson B., Neville E., Muck A., et al. Technical success and short-term results from mechanical thrombectomy for lower extremity iliofemoral deep vein thrombosis using a computer aided mechanical aspiration thrombectomy device. J Vasc Surg Venous Lymphat Disord. 2022;10(3):594–601. doi: 10.1016/j.jvsv.2021.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Lopez R., DeMartino R., Fleming M., Bjarnason H., Neisen M.J. Aspiration thrombectomy for acute iliofemoral or central deep venous thrombosis. J Vasc Surg Venous Lymphat Disord. 2019;7(2):162–168. doi: 10.1016/j.jvsv.2018.09.015. [DOI] [PubMed] [Google Scholar]
- 26.Fuller T., Neville E., Shapiro J., et al. Comparison of aspiration thrombectomy to other endovascular therapies for proximal upper extremity deep venous thrombosis. J Vasc Surg Venous Lymphat Disord. 2022;10(2):300–305. doi: 10.1016/j.jvsv.2021.07.017. [DOI] [PubMed] [Google Scholar]
- 27.Hamandi M., Lanfear A.T., Woolbert S., et al. Challenging management of a patient with severe bilateral deep vein thrombosis. J Investig Med High Impact Case Rep. 2020;8 doi: 10.1177/2324709620910288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Premier . Premier; 2021. PINC AI healthcare data: data that informs and performs (white paper). PINC AI Applied Sciences.https://offers.premierinc.com/rs/381-NBB-525/images/Premier-Healthcare-Database-Whitepaper-Final.pdf [Google Scholar]
- 29.Ribic C., Crowther M. Thrombosis and anticoagulation in the setting of renal or liver disease. Hematology Am Soc Hematol Educ Program. 2016;2016(1):188–195. doi: 10.1182/asheducation-2016.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khorana A.A., Mackman N., Falanga A., et al. Cancer-associated venous thromboembolism. Nat Rev Dis Primers. 2022;8(1):11. doi: 10.1038/s41572-022-00336-y. [DOI] [PubMed] [Google Scholar]
- 31.Hansson P.O., Sorbo J., Eriksson H. Recurrent venous thromboembolism after deep vein thrombosis: incidence and risk factors. Arch Intern Med. 2000;160(6):769–774. doi: 10.1001/archinte.160.6.769. [DOI] [PubMed] [Google Scholar]
- 32.Yeh Y.T., Tsai S.E., Chen Y.C., et al. Deep venous thrombosis and risk of consequent sepsis event: a retrospective nationwide ppopulation-based cohort study. Int J Environ Res Public Health. 2021;18(15):7879. doi: 10.3390/ijerph18157879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Katsoularis I., Fonseca-Rodriguez O., Farrington P., et al. Risks of deep vein thrombosis, pulmonary embolism, and bleeding after covid-19: nationwide self-controlled cases series and matched cohort study. BMJ. 2022;377 doi: 10.1136/bmj-2021-069590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Broderick C., Watson L., Armon M.P. Thrombolytic strategies versus standard anticoagulation for acute deep vein thrombosis of the lower limb. Cochrane Database Syst Rev. 2021;1(1) doi: 10.1002/14651858.CD002783.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xing Z., Tang L., Zhu Z., Hu X. Effects of thrombolysis on outcomes of patients with deep venous thrombosis: an updated meta-analysis. PLoS One. 2018;13(9) doi: 10.1371/journal.pone.0204594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murphy G.J., Reeves B.C., Rogers C.A., Rizvi S.I., Culliford L., Angelini G.D. Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery. Circulation. 2007;116(22):2544–2552. doi: 10.1161/CIRCULATIONAHA.107.698977. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.