Abstract
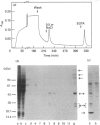
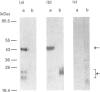
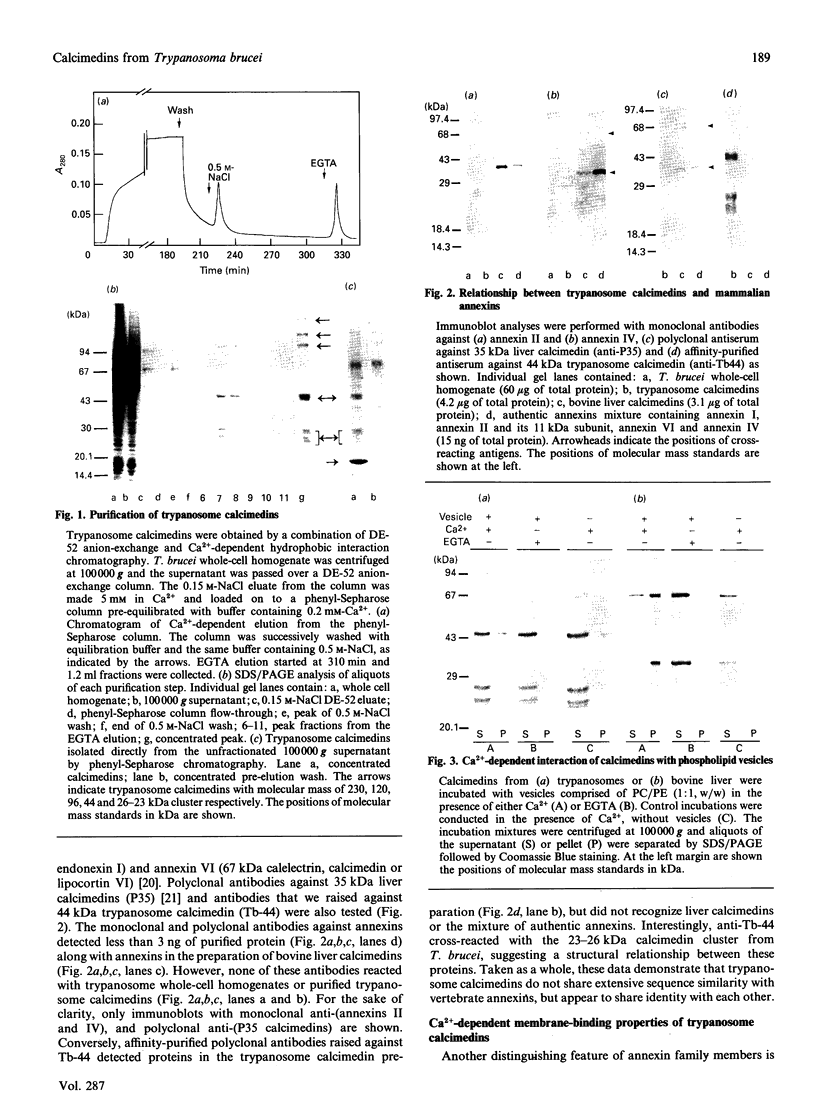
The cellular complement of calcimedins was identified in Trypanosoma brucei by Ca(2+)-dependent association with phenyl-Sepharose. Predominant calcimedins with molecular mass of 23-26 kDa and 44 kDa, along with minor calcimedins of 96, 120 and 230 kDa, were obtained. The trypanosome calcimedins were unrelated to vertebrate annexins, based upon antibody cross-reactivity and an inability to associate in a Ca(2+)-dependent way with phospholipid vesicles comprised of phosphatidylserine or phosphatidylethanolamine/phosphatidylcholine (1:1, w/w). Partial sequence analysis demonstrated that 44 kDa calcimedin (Tb-44) contained an EF-hand calcium-binding loop. Five CNBr/tryptic fragments exhibited a total of 93% similarity with Tb-17, a 23 kDa EF-hand protein in T. brucei. The trypanosome calcimedins appeared to comprise a family of proteins, based on sequence similarities and antibody cross-reactivity of affinity-purified anti-Tb44 with the 23-26 kDa cluster. No evidence was found for Tb-44 in the related species T. cruzi, Leishmania taraentolae or Crithidia fasciculata. Antibodies against Tb-44 were localized by immunofluorescence along the flagellum of T. brucei. Immunoblot analysis of flagella-enriched preparations demonstrated that Tb-44 and the 23-26 kDa cluster were present in this structure. We conclude that annexin family members are not among the predominant trypanosome proteins that associate with phenyl-Sepharose in a Ca(2+)-dependent way. Instead, the major trypanosome calcimedins comprise a family of flagellar EF-hand calcium-binding proteins.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali S. M., Geisow M. J., Burgoyne R. D. A role for calpactin in calcium-dependent exocytosis in adrenal chromaffin cells. Nature. 1989 Jul 27;340(6231):313–315. doi: 10.1038/340313a0. [DOI] [PubMed] [Google Scholar]
- Balber A. E. Trypanosoma brucei: fluxes of the morphological variants in intact and X-irradiated mice. Exp Parasitol. 1972 Apr;31(2):307–319. doi: 10.1016/0014-4894(72)90122-1. [DOI] [PubMed] [Google Scholar]
- Blackwood R. A., Ernst J. D. Characterization of Ca2(+)-dependent phospholipid binding, vesicle aggregation and membrane fusion by annexins. Biochem J. 1990 Feb 15;266(1):195–200. doi: 10.1042/bj2660195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne R. D., Geisow M. J. The annexin family of calcium-binding proteins. Review article. Cell Calcium. 1989 Jan;10(1):1–10. doi: 10.1016/0143-4160(89)90038-9. [DOI] [PubMed] [Google Scholar]
- Coppens I., Baudhuin P., Opperdoes F. R., Courtoy P. J. Receptors for the host low density lipoproteins on the hemoflagellate Trypanosoma brucei: purification and involvement in the growth of the parasite. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6753–6757. doi: 10.1073/pnas.85.18.6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crumpton M. J., Dedman J. R. Protein terminology tangle. Nature. 1990 May 17;345(6272):212–212. doi: 10.1038/345212a0. [DOI] [PubMed] [Google Scholar]
- Engman D. M., Krause K. H., Blumin J. H., Kim K. S., Kirchhoff L. V., Donelson J. E. A novel flagellar Ca2+-binding protein in trypanosomes. J Biol Chem. 1989 Nov 5;264(31):18627–18631. [PubMed] [Google Scholar]
- Feinberg J., Weinman J., Weinman S., Walsh M. P., Harricane M. C., Gabrion J., Demaille J. G. Immunocytochemical and biochemical evidence for the presence of calmodulin in bull sperm flagellum. Isolation and characterization of sperm calmodulin. Biochim Biophys Acta. 1981 Mar 18;673(3):303–311. doi: 10.1016/0304-4165(81)90338-x. [DOI] [PubMed] [Google Scholar]
- Gerke V. Consensus peptide antibodies reveal a widespread occurrence of Ca2+/lipid-binding proteins of the annexin family. FEBS Lett. 1989 Dec 4;258(2):259–262. doi: 10.1016/0014-5793(89)81668-0. [DOI] [PubMed] [Google Scholar]
- Gray M. A., Ross C. A., Taylor A. M., Luckins A. G. In vitro cultivation of Trypanosoma congolense: the production of infective metacyclic trypanosomes in cultures initiated from cloned stocks. Acta Trop. 1984 Dec;41(4):343–353. [PubMed] [Google Scholar]
- Haghighat N. G., Ruben L. Purification of novel calcium binding proteins from Trypanosoma brucei: properties of 22-, 24- and 38-kilodalton proteins. Mol Biochem Parasitol. 1992 Mar;51(1):99–110. doi: 10.1016/0166-6851(92)90205-x. [DOI] [PubMed] [Google Scholar]
- Hazarika P., Sheldon A., Kaetzel M. A., Díaz-Muñoz M., Hamilton S. L., Dedman J. R. Regulation of the sarcoplasmic reticulum Ca(2+)-release channel requires intact annexin VI. J Cell Biochem. 1991 May;46(1):86–93. doi: 10.1002/jcb.240460113. [DOI] [PubMed] [Google Scholar]
- Hinrichsen R. D., Burgess-Cassler A., Soltvedt B. C., Hennessey T., Kung C. Restoration by calmodulin of a Ca2+-dependent K+ current missing in a mutant of Paramecium. Science. 1986 Apr 25;232(4749):503–506. doi: 10.1126/science.2421410. [DOI] [PubMed] [Google Scholar]
- Holwill M. E., McGregor J. L. Control of flagellar wave movement in Crithidia oncopelti. Nature. 1975 May 8;255(5504):157–158. doi: 10.1038/255157a0. [DOI] [PubMed] [Google Scholar]
- Jamieson G. A., Jr, Vanaman T. C., Blum J. J. Presence of calmodulin in Tetrahymena. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6471–6475. doi: 10.1073/pnas.76.12.6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaetzel M. A., Dedman J. R. Affinity-purified site-directed antibody recognizes the entire annexin protein family. Biochem Biophys Res Commun. 1989 May 15;160(3):1233–1237. doi: 10.1016/s0006-291x(89)80135-4. [DOI] [PubMed] [Google Scholar]
- Keith K., Hide G., Tait A. Characterisation of protein kinase C like activities in Trypanosoma brucei. Mol Biochem Parasitol. 1990 Nov;43(1):107–116. doi: 10.1016/0166-6851(90)90135-9. [DOI] [PubMed] [Google Scholar]
- Kikkawa U., Kishimoto A., Nishizuka Y. The protein kinase C family: heterogeneity and its implications. Annu Rev Biochem. 1989;58:31–44. doi: 10.1146/annurev.bi.58.070189.000335. [DOI] [PubMed] [Google Scholar]
- Lee M. G., Chen J. F., Ho A. W., D'Alesandro P. A., Van der Ploeg L. H. A putative flagellar Ca2(+)-binding protein of the flagellum of trypanosomatid protozoan parasites. Nucleic Acids Res. 1990 Jul 25;18(14):4252–4252. doi: 10.1093/nar/18.14.4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore P. B., Dedman J. R. Calcium binding proteins and cellular regulation. Life Sci. 1982 Dec 27;31(26):2937–2946. doi: 10.1016/0024-3205(82)90059-5. [DOI] [PubMed] [Google Scholar]
- Moore P. B., Dedman J. R. Calcium-dependent protein binding to phenothiazine columns. J Biol Chem. 1982 Aug 25;257(16):9663–9667. [PubMed] [Google Scholar]
- Nagao S., Suzuki Y., Watanabe Y., Nozawa Y. Activation by a calcium-binding protein of guanylate cyclase in Tetrahymena pyriformis. Biochem Biophys Res Commun. 1979 Sep 12;90(1):261–268. doi: 10.1016/0006-291x(79)91619-x. [DOI] [PubMed] [Google Scholar]
- Olmsted J. B. Affinity purification of antibodies from diazotized paper blots of heterogeneous protein samples. J Biol Chem. 1981 Dec 10;256(23):11955–11957. [PubMed] [Google Scholar]
- Pepinsky R. B., Tizard R., Mattaliano R. J., Sinclair L. K., Miller G. T., Browning J. L., Chow E. P., Burne C., Huang K. S., Pratt D. Five distinct calcium and phospholipid binding proteins share homology with lipocortin I. J Biol Chem. 1988 Aug 5;263(22):10799–10811. [PubMed] [Google Scholar]
- Reeves J. P., Dowben R. M. Formation and properties of thin-walled phospholipid vesicles. J Cell Physiol. 1969 Feb;73(1):49–60. doi: 10.1002/jcp.1040730108. [DOI] [PubMed] [Google Scholar]
- Ross T. S., Tait J. F., Majerus P. W. Identity of inositol 1,2-cyclic phosphate 2-phosphohydrolase with lipocortin III. Science. 1990 May 4;248(4955):605–607. doi: 10.1126/science.2159184. [DOI] [PubMed] [Google Scholar]
- Ruben L., Akins C. D. Trypanosoma brucei: the tumor promoter thapsigargin stimulates calcium release from an intracellular compartment in slender bloodstream forms. Exp Parasitol. 1992 May;74(3):332–339. doi: 10.1016/0014-4894(92)90157-6. [DOI] [PubMed] [Google Scholar]
- Ruben L., Egwuagu C., Patton C. L. African trypanosomes contain calmodulin which is distinct from host calmodulin. Biochim Biophys Acta. 1983 Jul 29;758(2):104–113. doi: 10.1016/0304-4165(83)90290-8. [DOI] [PubMed] [Google Scholar]
- Ruben L., Hutchinson A., Moehlman J. Calcium homeostasis in Trypanosoma brucei. Identification of a pH-sensitive non-mitochondrial calcium pool. J Biol Chem. 1991 Dec 25;266(36):24351–24358. [PubMed] [Google Scholar]
- Ruben L., Patton C. L. Calmodulin from Trypanosoma brucei: immunological analysis and genomic organization. Methods Enzymol. 1987;139:262–276. doi: 10.1016/0076-6879(87)39091-3. [DOI] [PubMed] [Google Scholar]
- Ruben L., Ridgley E. L., Haghighat N. G., Chan E. Variant surface glycoprotein from Trypanosoma brucei clone YTat 1.1 contains a latent calmodulin-binding domain. Mol Biochem Parasitol. 1991 May;46(1):123–136. doi: 10.1016/0166-6851(91)90206-l. [DOI] [PubMed] [Google Scholar]
- Russell D. G., Newsam R. J., Palmer G. C., Gull K. Structural and biochemical characterisation of the paraflagellar rod of Crithidia fasciculata. Eur J Cell Biol. 1983 Mar;30(1):137–143. [PubMed] [Google Scholar]
- Smallwood M., Keen J. N., Bowles D. J. Purification and partial sequence analysis of plant annexins. Biochem J. 1990 Aug 15;270(1):157–161. doi: 10.1042/bj2700157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith V. L., Dedman J. R. An immunological comparison of several novel calcium-binding proteins. J Biol Chem. 1986 Dec 5;261(34):15815–15818. [PubMed] [Google Scholar]
- Strynadka N. C., James M. N. Crystal structures of the helix-loop-helix calcium-binding proteins. Annu Rev Biochem. 1989;58:951–998. doi: 10.1146/annurev.bi.58.070189.004511. [DOI] [PubMed] [Google Scholar]
- Südhof T. C., Ebbecke M., Walker J. H., Fritsche U., Boustead C. Isolation of mammalian calelectrins: a new class of ubiquitous Ca2+-regulated proteins. Biochemistry. 1984 Mar 13;23(6):1103–1109. doi: 10.1021/bi00301a010. [DOI] [PubMed] [Google Scholar]
- Tash J. S., Krinks M., Patel J., Means R. L., Klee C. B., Means A. R. Identification, characterization, and functional correlation of calmodulin-dependent protein phosphatase in sperm. J Cell Biol. 1988 May;106(5):1625–1633. doi: 10.1083/jcb.106.5.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorheis H. P., Martin B. R. Characteristics of the calcium-mediated mechanism activating adenylate cyclase in Trypanosoma brucei. Eur J Biochem. 1981 Jun 1;116(3):471–477. doi: 10.1111/j.1432-1033.1981.tb05360.x. [DOI] [PubMed] [Google Scholar]
- Walker J. H. Isolation from cholinergic synapses of a protein that binds to membranes in a calcium-dependent manner. J Neurochem. 1982 Sep;39(3):815–823. doi: 10.1111/j.1471-4159.1982.tb07965.x. [DOI] [PubMed] [Google Scholar]
- Walsh M. P., Valentine K. A., Ngai P. K., Carruthers C. A., Hollenberg M. D. Ca2+-dependent hydrophobic-interaction chromatography. Isolation of a novel Ca2+-binding protein and protein kinase C from bovine brain. Biochem J. 1984 Nov 15;224(1):117–127. doi: 10.1042/bj2240117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster P. Endocytosis by African trypanosomes. I. Three-dimensional structure of the endocytic organelles in Trypanosoma brucei and T. congolense. Eur J Cell Biol. 1989 Aug;49(2):295–302. [PubMed] [Google Scholar]
- Webster P., Grab D. J. Intracellular colocalization of variant surface glycoprotein and transferrin-gold in Trypanosoma brucei. J Cell Biol. 1988 Feb;106(2):279–288. doi: 10.1083/jcb.106.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa H., Furuki J., Takahashi Y., Morioka H., Yoshida Y. Freeze-fracture study of the bloodstream form of Trypanosoma brucei gambiense. J Protozool. 1990 Jan-Feb;37(1):27–32. doi: 10.1111/j.1550-7408.1990.tb01109.x. [DOI] [PubMed] [Google Scholar]
- da Cunha e Silva N. L., Hassón-Voloch A., de Souza W. Isolation and characterization of a highly purified flagellar membrane fraction from trypanosomatids. Mol Biochem Parasitol. 1989 Nov;37(1):129–136. doi: 10.1016/0166-6851(89)90109-6. [DOI] [PubMed] [Google Scholar]