Abstract
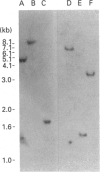
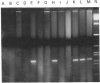
We have previously reported the cloning of cDNAs for a flavin-containing mono-oxygenase (FMO) of man, designated FMO1 [Dolphin, Shephard, Povey, Palmer, Ziegler, Ayesh, Smith & Phillips (1991) J. Biol. Chem. 266, 12379-12385], that is the orthologue of pig and rabbit hepatic FMOs. We now describe the isolation and characterization of cDNA clones for a second human FMO, which we have designated FMO2. The polypeptide encoded by the cDNAs is 558 amino acid residues long, has a calculated M(r) of 63337, and contains putative FAD- and NADP-binding sites that align exactly with those described in other mammalian FMOs. Human FMO2 has 51-53% primary sequence identity with human FMO1, rabbit pulmonary FMO and rabbit liver FMO form 2, and thus represents a fourth, distinct, member of the mammalian FMO family. The corresponding mRNA is present in low abundance in adult human liver. Southern blot hybridization with single-exon probes demonstrated that human FMO2 and FMO1 are the products of single genes. The gene encoding FMO2 (designated FMO2) was mapped, by the polymerase chain reaction, to human chromosome 1, the same chromosome on which FMO1 is located.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Waiz M., Ayesh R., Mitchell S. C., Idle J. R., Smith R. L. Trimethylaminuria (fish-odour syndrome): an inborn error of oxidative metabolism. Lancet. 1987 Mar 14;1(8533):634–635. doi: 10.1016/s0140-6736(87)90280-7. [DOI] [PubMed] [Google Scholar]
- Andrews P. W., Knowles B. B., Parkar M., Pym B., Stanley K., Goodfellow P. N. A human cell-surface antigen defined by a monoclonal antibody and controlled by a gene on human chromosome 1. Ann Hum Genet. 1985 Jan;49(Pt 1):31–39. doi: 10.1111/j.1469-1809.1985.tb01673.x. [DOI] [PubMed] [Google Scholar]
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- Dolphin C., Shephard E. A., Povey S., Palmer C. N., Ziegler D. M., Ayesh R., Smith R. L., Phillips I. R. Cloning, primary sequence, and chromosomal mapping of a human flavin-containing monooxygenase (FMO1). J Biol Chem. 1991 Jul 5;266(19):12379–12385. [PubMed] [Google Scholar]
- Edwards Y. H., Parkar M., Povey S., West L. F., Parrington J. M., Solomon E. Human myosin heavy chain genes assigned to chromosome 17 using a human cDNA clone as probe. Ann Hum Genet. 1985 May;49(Pt 2):101–109. doi: 10.1111/j.1469-1809.1985.tb01681.x. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Flinta C., Persson B., Jörnvall H., von Heijne G. Sequence determinants of cytosolic N-terminal protein processing. Eur J Biochem. 1986 Jan 2;154(1):193–196. doi: 10.1111/j.1432-1033.1986.tb09378.x. [DOI] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Gasser R., Tynes R. E., Lawton M. P., Korsmeyer K. K., Ziegler D. M., Philpot R. M. The flavin-containing monooxygenase expressed in pig liver: primary sequence, distribution, and evidence for a single gene. Biochemistry. 1990 Jan 9;29(1):119–124. doi: 10.1021/bi00453a014. [DOI] [PubMed] [Google Scholar]
- Guan S. H., Falick A. M., Cashman J. R. N-terminus determination: FAD and NADP binding domain mapping of hog liver flavin-containing monooxygenase by tandem mass spectrometry. Biochem Biophys Res Commun. 1990 Jul 31;170(2):937–943. doi: 10.1016/0006-291x(90)92181-x. [DOI] [PubMed] [Google Scholar]
- Hlavica P., Kehl M. Studies on the mechanism of hepatic microsomal N-oxide formation. The role of cytochrome P-450 and mixed-function amine oxidase in the N-oxidation of NN-dimethylaniline. Biochem J. 1977 Jun 15;164(3):487–496. doi: 10.1042/bj1640487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humbert J. A., Hammond K. B., Hathaway W. E. Trimethylaminuria: the fish-odour syndrome. Lancet. 1970 Oct 10;2(7676):770–771. doi: 10.1016/s0140-6736(70)90241-2. [DOI] [PubMed] [Google Scholar]
- Kielty C. M., Povey S., Hopkinson D. A. Regulation of expression of liver-specific enzymes. III. Further analysis of a series of rat hepatoma X human somatic cell hybrids. Ann Hum Genet. 1982 Oct;46(Pt 4):307–327. doi: 10.1111/j.1469-1809.1982.tb01582.x. [DOI] [PubMed] [Google Scholar]
- Kimura T., Kodama M., Nagata C. Purification of mixed-function amine oxidase from rat liver microsomes. Biochem Biophys Res Commun. 1983 Jan 27;110(2):640–645. doi: 10.1016/0006-291x(83)91197-x. [DOI] [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987 Oct 26;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lawton M. P., Gasser R., Tynes R. E., Hodgson E., Philpot R. M. The flavin-containing monooxygenase enzymes expressed in rabbit liver and lung are products of related but distinctly different genes. J Biol Chem. 1990 Apr 5;265(10):5855–5861. [PubMed] [Google Scholar]
- Lawton M. P., Kronbach T., Johnson E. F., Philpot R. M. Properties of expressed and native flavin-containing monooxygenases: evidence of multiple forms in rabbit liver and lung. Mol Pharmacol. 1991 Nov;40(5):692–698. [PubMed] [Google Scholar]
- Ozols J. Covalent structure of liver microsomal flavin-containing monooxygenase form 1. J Biol Chem. 1990 Jun 25;265(18):10289–10299. [PubMed] [Google Scholar]
- Ozols J. Liver microsomes contain two distinct NADPH-Monooxygenases with NH2-terminal segments homologous to the flavin containing NADPH-monooxygenase of Pseudomonas fluorescens. Biochem Biophys Res Commun. 1989 Aug 30;163(1):49–55. doi: 10.1016/0006-291x(89)92097-4. [DOI] [PubMed] [Google Scholar]
- Ozols J. Multiple forms of liver microsomal flavin-containing monooxygenases: complete covalent structure of form 2. Arch Biochem Biophys. 1991 Oct;290(1):103–115. doi: 10.1016/0003-9861(91)90596-b. [DOI] [PubMed] [Google Scholar]
- Sabourin P. J., Smyser B. P., Hodgson E. Purification of the flavin-containing monooxygenase from mouse and pig liver microsomes. Int J Biochem. 1984;16(7):713–720. doi: 10.1016/0020-711x(84)90180-0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelley E. D., Shelley W. B. The fish odor syndrome. Trimethylaminuria. JAMA. 1984 Jan 13;251(2):253–255. [PubMed] [Google Scholar]
- Solomon E., Swallow D., Burgess S., Evans L. Assignment of the human acid alpha-glucosidase gene (alphaGLU) to chromosome 17 using somatic cell hybrids. Ann Hum Genet. 1979 Jan;42(3):273–281. doi: 10.1111/j.1469-1809.1979.tb00661.x. [DOI] [PubMed] [Google Scholar]
- Tynes R. E., Sabourin P. J., Hodgson E., Philpot R. M. Formation of hydrogen peroxide and N-hydroxylated amines catalyzed by pulmonary flavin-containing monooxygenases in the presence of primary alkylamines. Arch Biochem Biophys. 1986 Dec;251(2):654–664. doi: 10.1016/0003-9861(86)90375-9. [DOI] [PubMed] [Google Scholar]
- Venkatesh K., Levi P. E., Hodgson E. The flavin-containing monooxygenase of mouse kidney. A comparison with the liver enzyme. Biochem Pharmacol. 1991 Sep 12;42(7):1411–1420. doi: 10.1016/0006-2952(91)90453-c. [DOI] [PubMed] [Google Scholar]
- Williams D. E., Ziegler D. M., Nordin D. J., Hale S. E., Masters B. S. Rabbit lung flavin-containing monooxygenase is immunochemically and catalytically distinct from the liver enzyme. Biochem Biophys Res Commun. 1984 Nov 30;125(1):116–122. doi: 10.1016/s0006-291x(84)80342-3. [DOI] [PubMed] [Google Scholar]
- Wong Z., Wilson V., Patel I., Povey S., Jeffreys A. J. Characterization of a panel of highly variable minisatellites cloned from human DNA. Ann Hum Genet. 1987 Oct;51(Pt 4):269–288. doi: 10.1111/j.1469-1809.1987.tb01062.x. [DOI] [PubMed] [Google Scholar]
- Ziegler D. M. Flavin-containing monooxygenases: catalytic mechanism and substrate specificities. Drug Metab Rev. 1988;19(1):1–32. doi: 10.3109/03602538809049617. [DOI] [PubMed] [Google Scholar]
- Ziegler D. M., Mitchell C. H. Microsomal oxidase. IV. Properties of a mixed-function amine oxidase isolated from pig liver microsomes. Arch Biochem Biophys. 1972 May;150(1):116–125. doi: 10.1016/0003-9861(72)90017-3. [DOI] [PubMed] [Google Scholar]