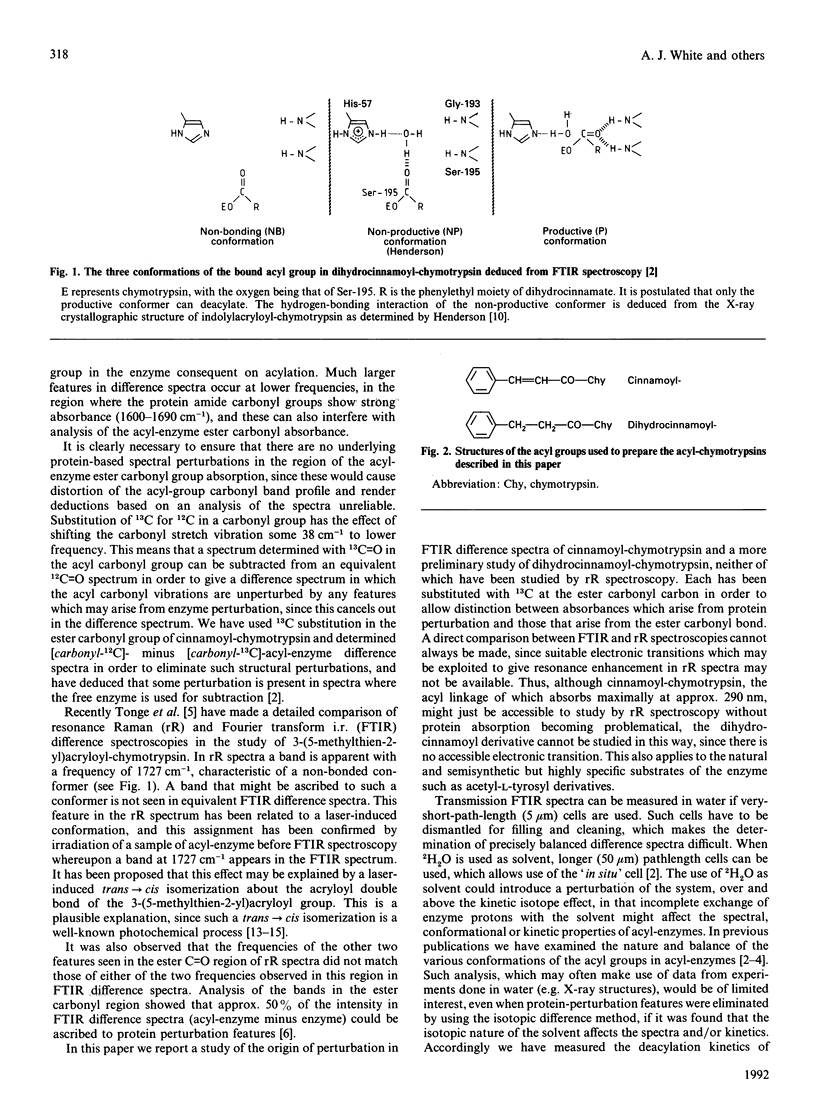
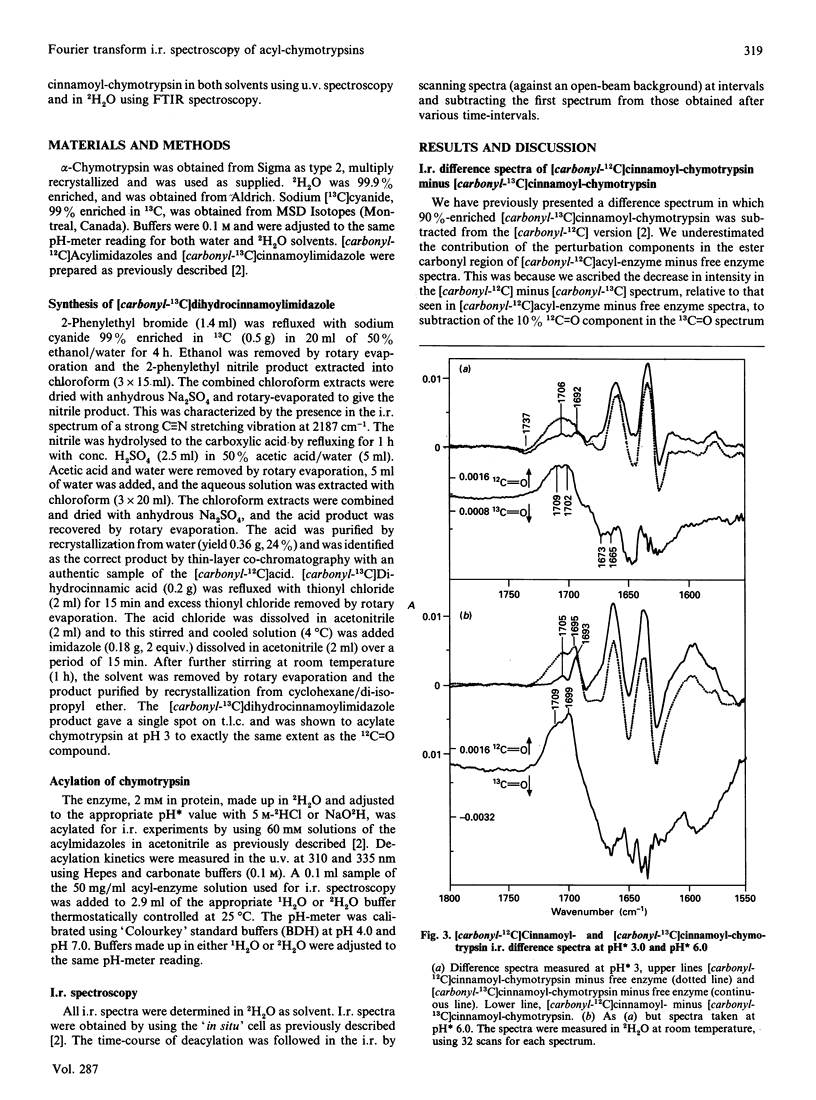
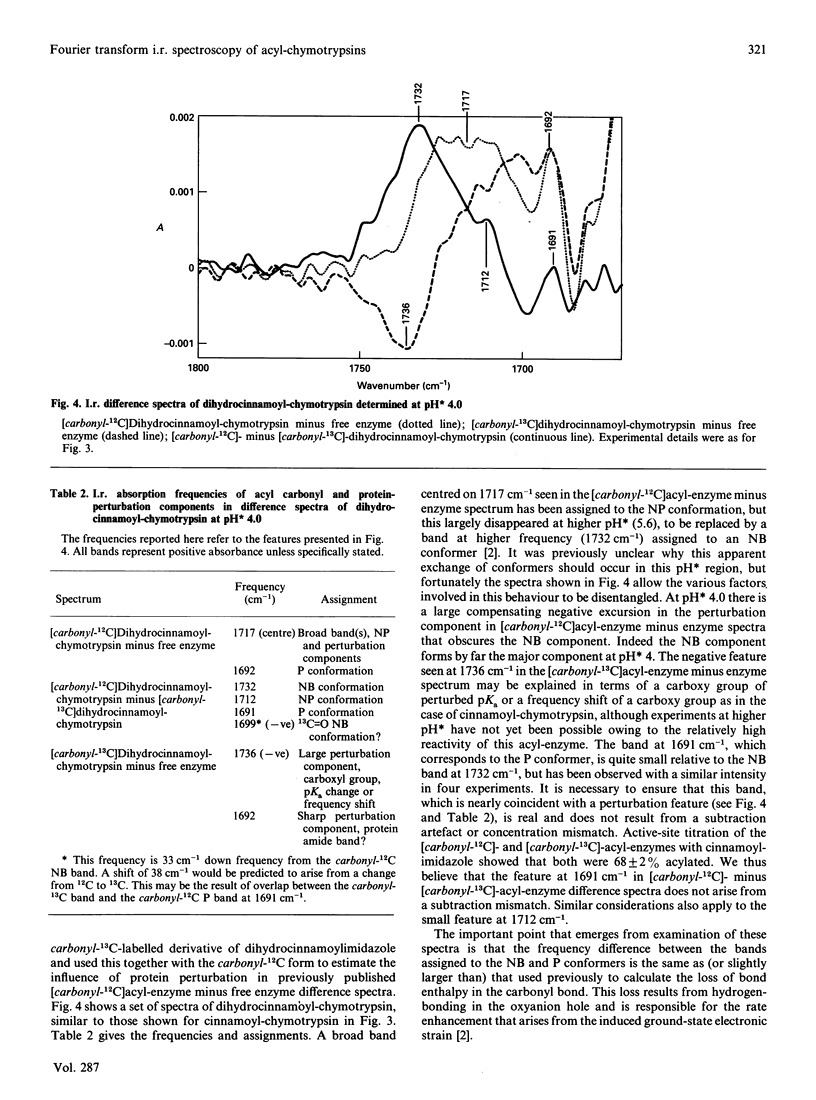
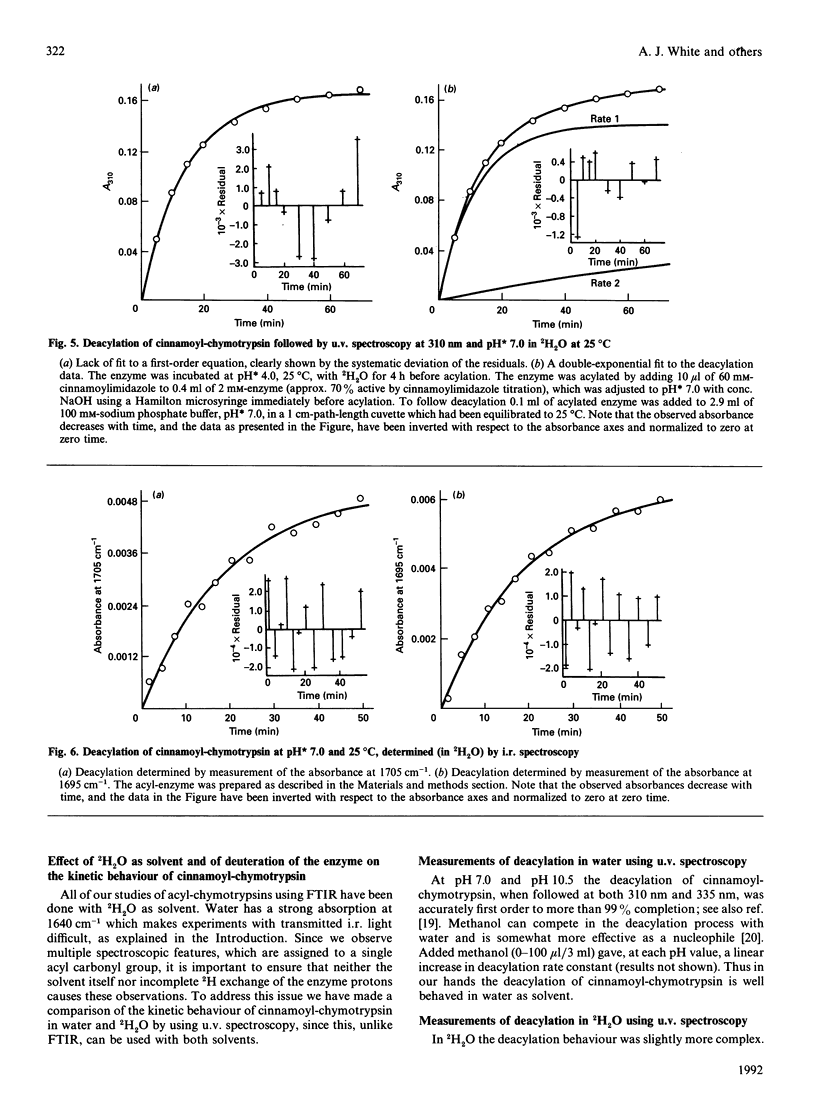
Abstract
I.r. spectroscopy has been applied to the study of hydrogen-bonding of the unique ester carbonyl group of acylchymotrypsins in the oxyanion hole of the enzyme. This catalytic device provides electrophilic stabilization of negative charge in the transition states and tetrahedral intermediates along the reaction pathway. The use of 13C isotope substitution of the ester carbonyl group reinforces the previous observation [White & Wharton (1990) Biochem. J. 270, 627-637] that the ester carbonyl group is significantly polarized in the ground state by hydrogen bonding in the oxyanion hole. I.r. difference spectra of [carbonyl-12C]-minus [carbonyl-13C]-cinnamoyl-chymotrypsin as well as each of these acylenzymes minus free enzyme are reported. These spectra show that the contribution of protein perturbation (i.e. spectral features that arise from the enzyme which is distorted on acylation) in [carbonyl-12C]cinnamoyl-chymotrypsin minus free enzyme spectra is significant. The contribution of the perturbation components of the spectra is pH-dependent and can represent up to 50% of the total absorbance in the spectral region from 1690 to 1740 cm-1. Use of the isotopic difference method has allowed problems associated with protein perturbation to be eliminated. Similar difference spectra are presented for dihydrocinnamoyl-chymotrypsin. In this case the effect of perturbation is very marked and leads to the cancellation of the band assigned to the non-bonded conformation of the acyl group which has previously only been observed at higher pH. The isotopic difference method again proves reliable and shows that the frequency difference previously used to calculate the ground-state electronic strain induced by the oxyanion-hole catalytic device is not affected by the perturbation, although the amplitudes of the spectral features are different. A study of the deacylation of cinnamoyl-chymotrypsin in water and deuterium oxide using both u.v. and i.r. spectroscopies has confirmed that the use of deuterium oxide as solvent has no serious effect on the deacylation behaviour of the enzyme. I.r. bands assigned to nonproductive and productive conformers decline identically during deacylation, which shows that the conformers are in dynamic exchange on the reaction time-scale.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belasco J. G., Knowles J. R. Direct observation of substrate distortion by triosephosphate isomerase using Fourier transform infrared spectroscopy. Biochemistry. 1980 Feb 5;19(3):472–477. doi: 10.1021/bi00544a012. [DOI] [PubMed] [Google Scholar]
- Deng H., Zheng J., Burgner J., Callender R. Molecular properties of pyruvate bound to lactate dehydrogenase: a Raman spectroscopic study. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4484–4488. doi: 10.1073/pnas.86.12.4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fersht A. R., Renard M. pH dependence of chymotrypsin catalysis. Appendix: substrate binding to dimeric alpha-chymotrypsin studied by x-ray diffraction and the equilibrium method. Biochemistry. 1974 Mar 26;13(7):1416–1426. doi: 10.1021/bi00704a016. [DOI] [PubMed] [Google Scholar]
- Fersht A. R., Sperling J. The charge relay system in chymotrypsin and chymotrypsinogen. J Mol Biol. 1973 Feb 25;74(2):137–149. doi: 10.1016/0022-2836(73)90103-4. [DOI] [PubMed] [Google Scholar]
- Henderson R. Structure of crystalline alpha-chymotrypsin. IV. The structure of indoleacryloyl-alpha-chyotrypsin and its relevance to the hydrolytic mechanism of the enzyme. J Mol Biol. 1970 Dec 14;54(2):341–354. doi: 10.1016/0022-2836(70)90434-1. [DOI] [PubMed] [Google Scholar]
- Kurz L. C., Drysdale G. R. Evidence from Fourier transform infrared spectroscopy for polarization of the carbonyl of oxaloacetate in the active site of citrate synthase. Biochemistry. 1987 May 5;26(9):2623–2627. doi: 10.1021/bi00383a032. [DOI] [PubMed] [Google Scholar]
- Tonge P. J., Carey P. R. Direct observation of the titration of substrate carbonyl groups in the active site of alpha-chymotrypsin by resonance Raman spectroscopy. Biochemistry. 1989 Aug 8;28(16):6701–6709. doi: 10.1021/bi00442a025. [DOI] [PubMed] [Google Scholar]
- Tonge P. J., Carey P. R. Length of the acyl carbonyl bond in acyl-serine proteases correlates with reactivity. Biochemistry. 1990 Dec 4;29(48):10723–10727. doi: 10.1021/bi00500a002. [DOI] [PubMed] [Google Scholar]
- Tonge P. J., Pusztai M., White A. J., Wharton C. W., Carey P. R. Resonance Raman and Fourier transform infrared spectroscopic studies of the acyl carbonyl group in [3-(5-methyl-2-thienyl)acryloyl]chymotrypsin: evidence for artifacts in the spectra obtained by both techniques. Biochemistry. 1991 May 14;30(19):4790–4795. doi: 10.1021/bi00233a021. [DOI] [PubMed] [Google Scholar]
- Tonge P., Moore G. R., Wharton C. W. Fourier-transform infra-red studies of the alkaline isomerization of mitochondrial cytochrome c and the ionization of carboxylic acids. Biochem J. 1989 Mar 1;258(2):599–605. doi: 10.1042/bj2580599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wharton C. W. Infra-red and Raman spectroscopic studies of enzyme structure and function. Biochem J. 1986 Jan 1;233(1):25–36. doi: 10.1042/bj2330025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White A. J., Drabble K., Wharton C. W. Fourier transform infrared spectroscopy of (13C=O) trans-cinnamoyl- and (13C=O) hydrocinnamoyl-a-chymotrypsins. Biochem Soc Trans. 1991 Apr;19(2):159S–159S. doi: 10.1042/bst019159s. [DOI] [PubMed] [Google Scholar]
- White A. J., Wharton C. W. Hydrogen-bonding in enzyme catalysis. Fourier-transform infrared detection of ground-state electronic strain in acyl-chymotrypsins and analysis of the kinetic consequences. Biochem J. 1990 Sep 15;270(3):627–637. doi: 10.1042/bj2700627. [DOI] [PMC free article] [PubMed] [Google Scholar]


