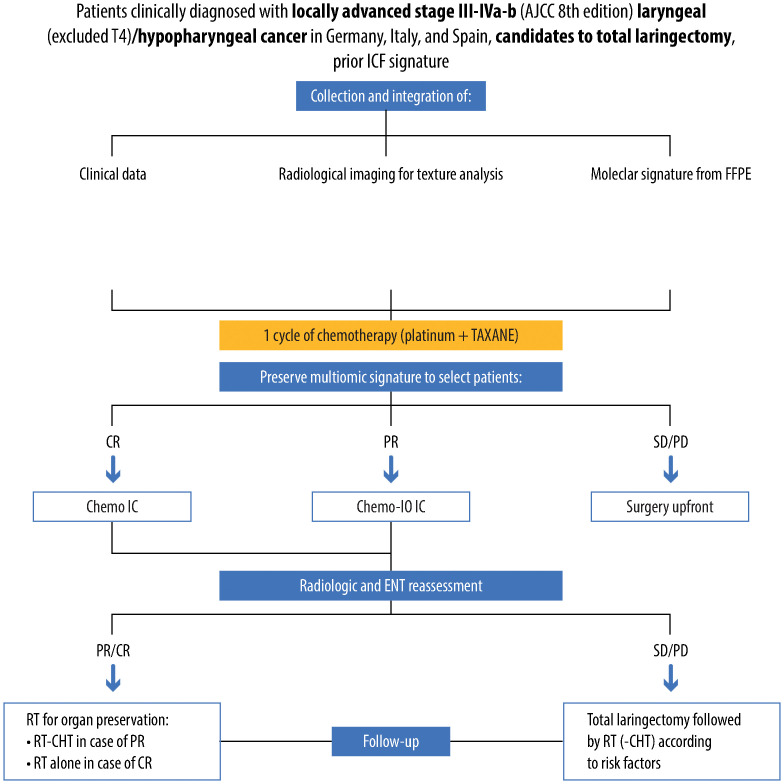
Figure 3.
Detailed design of the clinical PRESERVE trial, reporting selected population, methods of analysis, performed treatments and therapies, results and follow-up of patients. Specifically, patients affected by stage III-IV, non-metastatic, laryngeal/hypopharyngeal cancer candidates to total laryngectomy will be enrolled. For all patients, a multiomic assessment including clinical factors, radiomic and genomic analysis will be performed, providing a prediction of response to induction chemotherapy. Patients predicted with partial response (PR) will receive chemo-immunotherapy induction treatment. After three cycles, patients will undergo endoscopical and radiological restaging and receive curative treatment according to a tailored strategy, and subsequent follow-up.