Abstract
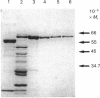
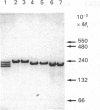
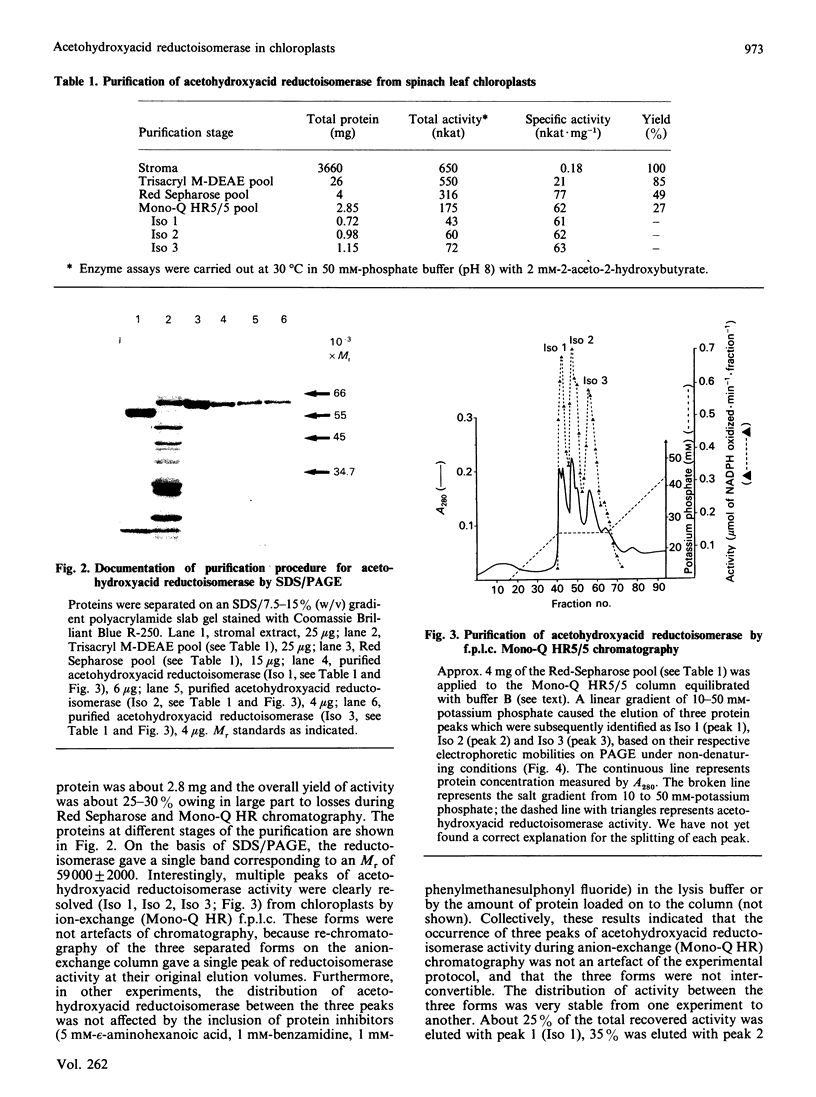
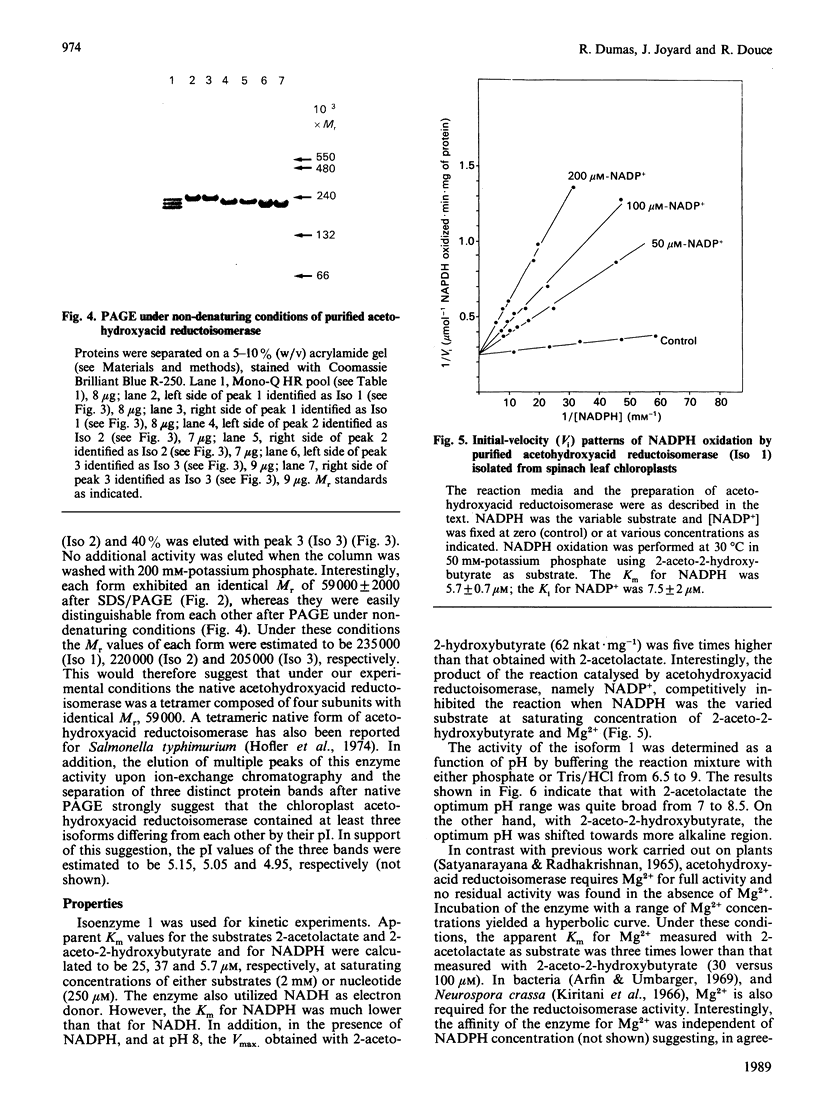
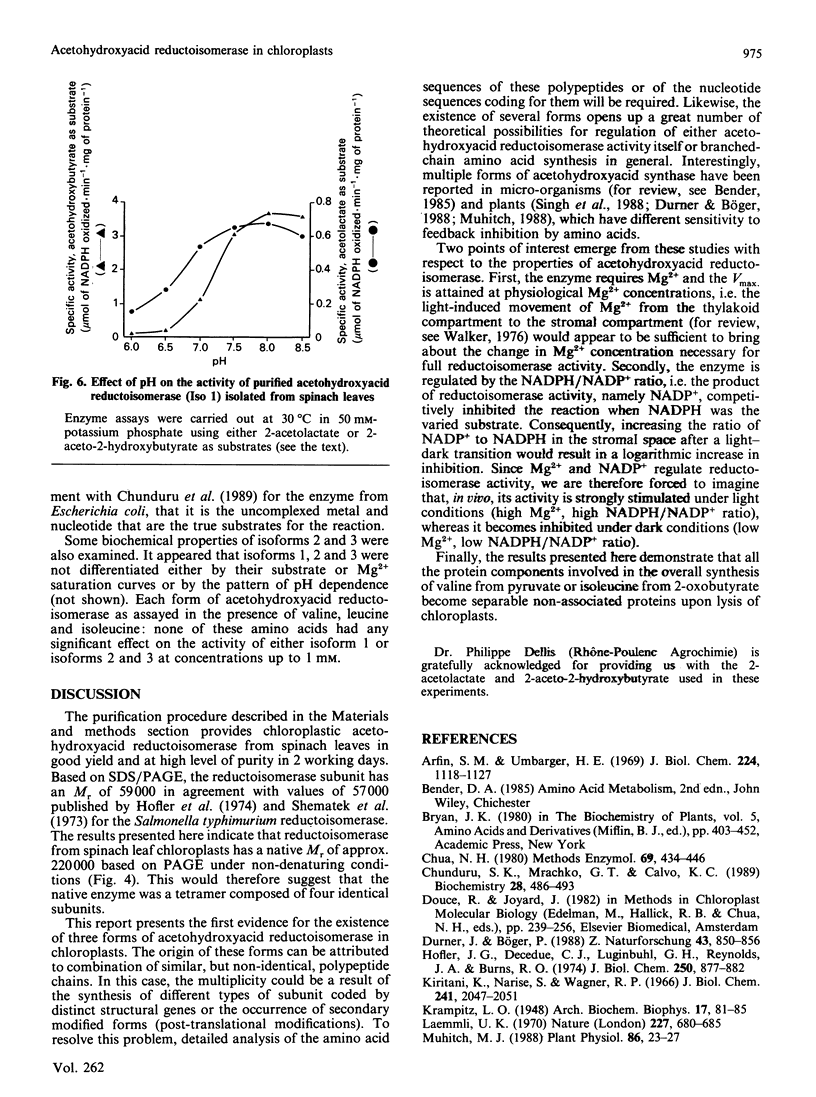
Acetohydroxyacid reductoisomerase was purified over 400-fold to a specific activity of 62 nkat.mg-1, with 2-aceto-2-hydroxybutyrate as substrate, from the stroma of spinach leaf chloroplasts. The enzyme was not intrinsically membrane bound. The native enzyme was a tetramer with a subunit Mr of 59,000. The activity was optimum between pH 7.5 and 8.5. The apparent Km for 2-acetolactate was 25 microM and for 2-aceto-2-hydroxybutyrate was 37 microM. The enzyme required Mg2+ and the Vmax. was attained at physiological Mg2+ concentrations. NADP+ competitively inhibited the reaction when NADPH was the varied substrate. The native enzyme eluted from Mono-Q ion-exchange resins as three distinct peaks of activity. This elution pattern was preserved when the peaks were combined, dialysed and re-chromatographed. Each form exhibited identical Mr of 59,000 after SDS/polyacrylamide gel electrophoresis (PAGE), whereas they were easily distinguishable from each other after PAGE under non-denaturing conditions. These results provide evidence for the existence of multiple forms of acetohydroxyacid reductoisomerase in chloroplasts isolated from spinach leaves.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arfin S. M., Umbarger H. E. Purification and properties of the acetohydroxy acid isomeroreductase of Salmonella typhimurium. J Biol Chem. 1969 Mar 10;244(5):1118–1127. [PubMed] [Google Scholar]
- Chunduru S. K., Mrachko G. T., Calvo K. C. Mechanism of ketol acid reductoisomerase--steady-state analysis and metal ion requirement. Biochemistry. 1989 Jan 24;28(2):486–493. doi: 10.1021/bi00428a012. [DOI] [PubMed] [Google Scholar]
- Hofler J. G., Decedue C. J., Luginbuhl G. H., Reynolds J. A., Burns R. O. The subunit structure of alpha-acetohydroxyacid isomeroreductase from Salmonella typhimurium. J Biol Chem. 1975 Feb 10;250(3):877–882. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Muhitch M. J. Acetolactate Synthase Activity in Developing Maize (Zea mays L.) Kernels. Plant Physiol. 1988 Jan;86(1):23–27. doi: 10.1104/pp.86.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primerano D. A., Burns R. O. Role of acetohydroxy acid isomeroreductase in biosynthesis of pantothenic acid in Salmonella typhimurium. J Bacteriol. 1983 Jan;153(1):259–269. doi: 10.1128/jb.153.1.259-269.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SATYANARAYANA T., RADHAKRISHNAN A. N. Biosynthesis of valine and isoleucine in plants. Biochim Biophys Acta. 1962 Jan 1;56:197–199. doi: 10.1016/0006-3002(62)90554-1. [DOI] [PubMed] [Google Scholar]
- Satyanarayana T., Radhakrishnan A. N. Biosynthesis of valine and isoleucine in plants. 3. Reductoisomerase of Phaseolus radiatus. Biochim Biophys Acta. 1965 Nov 22;110(2):380–388. doi: 10.1016/s0926-6593(65)80045-5. [DOI] [PubMed] [Google Scholar]
- Schulz A., Spönemann P., Köcher H., Wengenmayer F. The herbicidally active experimental compound Hoe 704 is a potent inhibitor of the enzyme acetolactate reductoisomerase. FEBS Lett. 1988 Oct 10;238(2):375–378. doi: 10.1016/0014-5793(88)80515-5. [DOI] [PubMed] [Google Scholar]
- Shematek E. M., Arfin S. M., Diven W. F. A kinetic study of -acetohydroxy acid isomeroreductase from Salmonella typhimurium. Arch Biochem Biophys. 1973 Sep;158(1):132–138. doi: 10.1016/0003-9861(73)90605-x. [DOI] [PubMed] [Google Scholar]