Abstract
Background
The presence of fibrotic interstitial lung disease (ILD) is relatively common in patients with emphysema. This has been designated combined pulmonary fibrosis and emphysema (CPFE). CPFE had worse prognosis than emphysema alone. Krebs von den Lungen-6 (KL-6) levels as a biomarker of alveolar type 2 epithelial cell injury, which is widely used to identify the presence of ILD, whether it can differentiate CPFE from COPD remains unknown.
Methods
259 patients from Xiangya Hospital with diagnosis of COPD, with or without ILD, and who had KL-6 tests were recruited for this retrospective analysis. Recorded data included demographic information, comorbidities, inflammatory biomarkers. Results of CT and pulmonary function tests were collected one week before or after KL-6 measurements.
Results
Among 259 patients, 52 patients were diagnosed with CPFE. The mean age was 67.39 ± 8.14 yeas. CPFE patients had higher ratio of rheumatic diseases (21.2 % vs 7.2 %, P = 0.003). CPFE patients exhibited higher values of FEV1 (1.97 vs 1.57, P = 0.002) and FEV1/FVC ratio (69.46 vs 57.64, P < 0.001) compared to COPD patients. CPFE patients had higher eosinophil counts, percentage of eosinophils, lactate dehydrogenase, total bilirubin levels and lower platelet counts. Serum KL-6 levels were higher in CPFE group compared to COPD group (574.95 vs 339.30 U/mL, P < 0.001). Multiple logistic regression showed that KL-6 level was an independent predictive factor for the presence of ILD among COPD patients. The AUC of serum KL-6 levels to differentiate CPFE was 0.711, with 95 % CI being 0.635 to 0.787. The cutoff point of KL-6 level was 550.95 U/mL with 57.7 % sensitivity and 79.7 % specificity for the discrimination of CPFE from COPD.
Conclusion
CPFE patients show higher KL-6 levels compared to isolated COPD, suggesting the potential of KL-6 as a practical screening tool for interstitial lung disease, specifically CPFE. A KL-6 threshold of 550.95 U/mL in COPD patients may indicate a high need for high-resolution chest computed tomography to detect fibrosis.
Keywords: COPD, CPFE, KL-6
Highlights
-
•
HRCT, the gold standard for diagnosing CPFE, is limited in its application due to cost and radiation concerns.
-
•
CPFE patients demonstrate significantly elevated KL-6 levels in comparison to individuals with isolated COPD.
-
•
A KL-6 threshold of 550.95 U/mL in COPD patients warrants HRCT evaluation to identify the presence of fibrosis.
1. Introduction
Tobacco smoke is one of the most important risk factors associated with the development of pulmonary diseases. Apart from chronic obstructive pulmonary disease (COPD), in some individuals, tobacco smoke can also trigger interstitial damage that results in pulmonary fibrosis, patients who had both emphysema and fibrosis were called combined pulmonary fibrosis and emphysema (CPFE) [1]. CPFE was first reported in 1948, the prevalence estimates of CPFE vary largely depending on the population recruited and the definition used, ranging from 8 to 67 %. Currently, there is a lack of comprehensive understanding of CPFE due to the absence of consensus on diagnostic criteria until 2022. As such, it is difficult to compare cohorts and draw consistent conclusions about the features, outcomes, and optimal management of these patients.
It is reported that patients with CPFE have worse survival than patients with emphysema alone [2]. Additionally, CPFE patients are more likely to have complications, including pulmonary artery hypertension and lung cancer [[3], [4], [5]], which may lead to worse outcomes. At present, high-resolution computed tomography (HRCT) is very important in differentiating fibrotic interstitial lung disease(fILD). However, it is challenging to perform routine screening among COPD patients due to the limitations of cost, radiation, and other considerations. Moreover, lung function tests were more frequently used in COPD management for motoring the disease progress, making it difficult to achieve early diagnosis of fILD. The identification of practical biomarkers for recognizing fibrotic ILD could help reduce economic costs and improve patient outcomes by enabling timely therapy.
Krebs von den Lungen-6 (KL-6) is classified as a polymorphic epithelial mucin (MUC1), representing a high molecular weight glycoprotein primarily secreted by injured bronchial epithelial cells or type II alveolar epithelial cells [6]. At present, KL-6 is widely used to screen early ILD, especially among patients with connective tissue disease(CTD) [7,8]. An optimal cutoff value of 500 U/mL has been established through comparisons between the interstitial pneumonia group and control groups consisting of other respiratory diseases, and is currently employed in certain countries as part of clinical practice [9]. COPD is a chronic inflammatory disease of the airways, epithelial damage plays a crucial role in its pathophysiology [10]. Given the elevated KL-6 levels found in ILD patients, more research is necessary to ascertain whether KL-6 can be useful in distinguishing CPFE patients from those with COPD. Additionally, with the new consensus on CPFE, more research is needed to explore its prevalence and clinical characteristics [1].
2. Methods
2.1. Study design and patient population
The present study obtained approval from the Ethics Committee of Xiangya Hospital, affiliated with Central South University, and was conducted in full compliance with the principles outlined in the Declaration of Helsinki and its subsequent revisions. The Ethics number assigned to this study is 202309183, and it was officially approved on September 6th, 2023. Detailed information pertaining to this study can be accessed at (https://ethics.tonoinfo.com/#/home/zndxxyyy). Informed consent was exempted due to the retrospective design of the study, and the analysis was performed using anonymized clinical data.
This study employed a retrospective design. The data were obtained from the database established by Xiangya Hospital, affiliated with Central South University, located in Hunan, China. The database included patients diagnosed with COPD who received medical care in the inpatient departments of Xiangya Hospital over a span of 20 years. Specifically, we focused on inpatients who had KL-6 measurements available in the comprehensive database. In cases where multiple KL-6 tests were conducted, we selected the first test administered upon admission. Subsequently, we performed a comprehensive search of the medical records using the keyword 'COPD' to verify that patients had a corresponding discharge diagnosis, and this diagnosis was further confirmed by two researchers. Following that, we proceed with the exclusion of specific infections that potentially impact KL6 levels, such as COVID-19, Pneumocystis jirovecii pneumonia (PJP), and tuberculosis. The CT images of these selected patients were meticulously reviewed by two pulmonologists and radiologists. Patients without CT data were excluded from the study. Based on the presence of pulmonary fibrosis, the recruited patients were categorized into two groups: the COPD group and the CPFE group. The diagnosis of emphysema was further validated by experienced radiologists and pulmonologists. The recorded data encompassed essential demographic information, such as age, gender, BMI, blood type, occupation, and smoking history. Additionally, regular blood biochemical tests, KL-6 levels, CT scans, and lung function parameters were collected. All data were documented upon admission, with CT scans and pulmonary function results obtained within one week of the KL-6 data.
2.2. KL-6 measurements
A 4 ml standardized blood sample was obtained from each patient according to established protocols. The serum was then separated via centrifugation at 3000 rpm for 10 min and stored at 4 °C until analysis. The KL-6 level (U/ml) was measured using the Nanopia® KL-6 kit (SEKISUI MEDICAL CO.LTD., Tokyo, Japan) through a latex particle-enhanced turbidimetric immunoassay (LETIA), following the manufacturer's instructions. In summary, KL-6 in the samples forms agglutination with latex particles coated with mouse KL-6 monoclonal antibodies through the antigen-antibody reaction. The resulting change in absorbance is measured to determine the KL-6 level. The automated analyzer used for the KL-6 assay has a measurement range of 50–5000 U/ml (r > 0.990). If the KL-6 concentration in a sample exceeds this range, it is diluted with a specific buffer containing pH 7.6, 0.025 mol/L N2 hydroxyethylpiperazine-N′-2-ethanesulfonic acid buffer, and 20 % newborn calf serum. These samples are recommended to be diluted up to 5 times, and the obtained KL-6 concentration is multiplied by the dilution factor to determine the original sample's KL-6 concentration. The assay demonstrated high repeatability with a coefficient of variation (CV) below 10 % and a relative deviation (B) under 15 %.
2.3. Diagnostic criteria of CPFE
We applied the definition of CPFE as recommended in the latest expert consensus [1]. CPFE is characterized by the coexistence of emphysema and interstitial fibrosis, exhibiting a diverse range of manifestations on high-resolution chest computed tomography (HRCT). Emphysema is identified as areas of reduced attenuation (also referred to as density) without visible walls on CT scans. Emphysematous foci can be classified as centrilobular, paraseptal, or panacinar. Interstitial fibrosis is recognized as regions of increased lung tissue attenuation, presenting as reticulation and/or ground-glass opacities, often accompanied by honeycombing and/or traction bronchiectasis. To meet the HRCT criteria for CPFE, patients must fulfill the following conditions: 1) Presence of emphysema, regardless of subtype, on HRCT, characterized by well-defined areas of low attenuation delineated by a very thin wall (<1 mm) or no wall, involving a minimum of 5 % of the total lung volume; 2) Presence of lung fibrosis, regardless of subtype.
2.4. Pulmonary function test
The pulmonary function test was conducted by skilled technicians using a spirometer (MasterScreen-Body/Diff, CareFusion, Germany) in accordance with the guidelines set forth by the American Thoracic Society. The spirometry procedures were carried out by fully trained and certified technicians with expertise in spirometry techniques. Spirometry data were included in the analysis if subjects had a minimum of three acceptable forced expiratory maneuvers and the differences between the highest values of two FEV1 and FVC measurements were within 5 % or 150 mL, in accordance with the acceptability and repeatability criteria outlined by the ATS/ERS [11]. The lung function prediction equations utilized in this study were derived from the global lung function 2012 equations [12], which serve as a widely recognized reference. To ensure accurate interpretation of results within the Chinese population, these equations were further adjusted to align with their specific characteristics [13]. The detailed predicted value equations employed in our analysis have been meticulously documented and are provided in Supplementary Table 1 for comprehensive reference.
2.5. Imaging evaluation of CT scan
All patients underwent a standard chest computed tomography (CT) scan utilizing one of our three CT scanners: a 16-MDCT (Brilliance 16, Philipps), a 64-MDCT (SOMATOM Definition, Siemens), or a 320-MDCT (Aquilion ONE, Toshiba Medical Systems) scanner. The imaging parameters for thin-section CT scans across different multidetector devices were as follows: tube voltage of 120 kV, automatic tube current modulation, matrix size of 512 x 512, and a slice thickness ranging from 1 to 1.5 mm. A board-certified radiologist and pulmonologist, both of whom were blinded to the clinical information, assessed the extent of emphysema and fibrosis (grade 1, 5–25 %; grade 2, 26%–50 %; grade 3, 51%–75 %; grade 4, 76%–100 %). Additionally, they evaluated the specific type of emphysema and the characteristics of the fibrotic lesions.
2.6. Statistical analysis
Continuous variables were presented as the mean and standard deviation if the data followed a normal distribution, and as the median and interquartile range (IQR) if the data did not exhibit a normal distribution. Categorical variables were described in terms of frequency rates and percentages. To compare means of continuous variables with normally distributed data, we employed the t-test or analysis of variance (ANOVA). For non-normally distributed data, we utilized non-parametric tests. The proportions of categorical variables were analyzed using the χ2 test. The optimal cutoff point on the receiver operating characteristic (ROC) curve was determined through the maximization of the Youden index. Statistical analyses were performed using SPSS (version 26.0; SPSS Company, Chicago, IL, United States) and the Free Statistics analysis platform. A significance level of P < 0.05 was considered statistically significant. Graphs were generated using GraphPad Prism version 9.00 software and the Free Statistics analysis platform.
3. Results
3.1. Demographic of the study population
A retrospective review was undertaken on a total of 311 patients who were diagnosed with COPD, all of whom had KL-6 measurements accessible in the database. Nonetheless, 52 patients were excluded from the study due to specific infections or inadequate CT data. Consequently, a final cohort of 259 patients was included in the analysis. Among these patients, 207 were diagnosed with simple COPD, while the remaining 52 were diagnosed with CPFE (Supplementary Fig. 1).
The study participants had a mean age of 67.39 years (SD: 8.14), with the majority being male (90.7 %). The average body mass index (BMI) was 22.63 kg/m2 (SD: 3.26). The most prevalent occupations among the patients were farmers (35.5 %) and retirees (31.3 %). Regarding smoking history, patients were categorized as current smokers (42.9 %), former smokers (46.0 %), or never smokers (11.1 %). Among current and former smokers, the median duration of cigarette consumption was 45.00 years (IQR: 30.00–60.00). There were no significant differences observed between the two groups in terms of age, gender, occupation, blood type and smoking status. Indeed, the prevalence of rheumatic diseases was found to be higher among CPFE patients in comparison to COPD patients (21.2 % vs. 7.2 %, P = 0.003). However, upon further subdivision of rheumatic diseases, no statistically significant difference was observed (Table 1).
Table 1.
Baseline and clinical characteristics between subjects with COPD and CPFE.
| Variable | Total (n = 259) | COPD (n = 207) | CPFE (n = 52) | P Value |
|---|---|---|---|---|
| Age, median (IQR), years | 67.39 ± 8.14 | 67.29 ± 8.16 | 67.79 ± 8.16 | 0.694 |
| Gender, n (%) | 0.182 | |||
| Female | 24 (9.3) | 22 (10.6) | 2 (3.8) | |
| Male | 235 (90.7) | 185 (89.4) | 50 (96.2) | |
| BMI | 22.63 ± 3.26 | 22.71 ± 3.12 | 22.40 ± 3.68 | 0.644 |
| Occupation, n (%) | 0.894 | |||
| Farmers | 92 (35.5) | 72 (34.8) | 20 (38.5) | |
| Employees | 30 (11.6) | 26 (12.6) | 4 (7.7) | |
| Freelancer | 7 (2.7) | 6 (2.9) | 1 (1.9) | |
| Retired | 81 (31.3) | 64 (30.9) | 17 (32.7) | |
| Unemployed | 19 (7.3) | 14 (6.8) | 5 (9.6) | |
| Other | 30 (11.6) | 25 (12.1) | 5 (9.6) | |
| Blood type (ABO), n (%) | 0.844 | |||
| A | 56 (35.2) | 47 (35.1) | 9 (36.0) | |
| B | 35 (22.0) | 30 (22.4) | 5 (20.0) | |
| O | 55 (34.6) | 47 (35.1) | 8 (32.0) | |
| AB | 13 (8.2) | 10 (7.5) | 3 (12.0) | |
| Smoking status, n (%) | 0.202 | |||
| Current smoker | 108 (42.9) | 85 (42.1) | 23 (46.0) | |
| Former smoker | 116 (46.0) | 91 (45.0) | 25 (50.0) | |
| Non-smoker | 28 (11.1) | 26 (12.9) | 2 (4.0) | |
| Cigarette consumption-pack years | 45.00 (30.00–60.00) | 45.00 (30.00–60.00) | 40.00 (30.00–72.75) | 0.941 |
| Comorbidities, n (%) | ||||
| Lung cancer | 129 (52.7) | 108 (55.1) | 21 (42.9) | 0.125 |
| Pulmonary hypertension | 53 (20.5) | 45 (21.7) | 8 (15.4) | 0.310 |
| Hypertension | 98 (37.8) | 79 (38.2) | 19 (36.5) | 0.829 |
| Coronary heart disease | 66 (25.5) | 54 (26.1) | 12 (23.1) | 0.656 |
| Cerebrovascular diseases | 25 (9.7) | 19 (9.2) | 6 (11.5) | 0.794 |
| Diabetes | 54 (20.8) | 40 (19.3) | 14 (26.9) | 0.228 |
| Tuberculosis | 79 (30.5) | 65 (31.4) | 14 (26.9) | 0.615 |
| Chronic liver diseases | 20 (7.7) | 17 (8.2) | 3 (5.8) | 0.773 |
| Rheumatic diseases | 26 (10.0) | 15 (7.2) | 11 (21.2) | 0.003 |
| Rheumatoid arthritis | 7 (2.7) | 4 (1.9) | 3 (5.8) | 0.147 |
| Systemic sclerosis | 4 (1.5) | 2 (1.0) | 2 (3.8) | 0.097 |
| ANCA-associated vasculitis | 5 (1.9) | 2 (1.0) | 3 (5.8) | 0.057 |
| Idiopathic inflammatory myositis | 1 (0.4) | 1 (0.5) | 0 (0) | 1.000 |
| Gout | 1 (0.4) | 1 (0.5) | 0 (0) | 1.000 |
| Systemic Lupus Erythematosus | 2 (0.8) | 2 (1.0) | 0 (0) | 1.000 |
| Osteoarthritis | 3 (1.2) | 2 (1.0) | 1 (1.9) | 0.491 |
| Mixed connective tissue disease | 3 (1.2) | 1 (0.5) | 2 (3.8) | 0.104 |
| Clinical Manifestations, n (%) | ||||
| Fever | 74 (28.7) | 58 (28.0) | 16 (31.4) | 0.635 |
| Cough | 225 (87.2) | 183 (88.4) | 42 (82.4) | 0.246 |
| Expectoration | 213 (82.6) | 174 (84.1) | 39 (76.5) | 0.201 |
| Hemoptysis | 32 (12.4) | 26 (12.6) | 6 (11.8) | 0.877 |
| Dyspnea | 176 (68.2) | 142 (68.6) | 34 (66.7) | 0.791 |
| Lung function | ||||
| FEV1, L | 1.67 ± 0.64 | 1.57 ± 0.62 | 1.97 ± 0.61 | 0.002 |
| FEV1, % predicted | 66.00 ± 21.58 | 62.99 ± 21.48 | 74.24 ± 19.93 | 0.012 |
| FVC, L | 2.73 ± 0.70 | 2.68 ± 0.66 | 2.87 ± 0.82 | 0.169 |
| FVC, % predicted | 82.88 ± 18.27 | 82.48 ± 17.85 | 83.96 ± 19.64 | 0.702 |
| FEV1/FVC, % | 60.62 ± 14.74 | 57.64 ± 14.24 | 69.46 ± 12.66 | <0.001 |
| DLCO, mmol/min/kPa | 3.95 ± 1.58 | 4.03 ± 1.73 | 3.73 ± 1.11 | 0.490 |
| DLCO, % predicted | 51.19 ± 18.90 | 52.81 ± 20.01 | 46.95 ± 15.29 | 0.253 |
| TLC, L | 4.46 ± 0.89 | 4.40 ± 0.86 | 4.62 ± 0.98 | 0.384 |
| Extent of emphysema lesion | 0.695 | |||
| 0 % | 14 (5.4) | 14 (6.8) | 0 (0.0) | |
| <5 % | 40 (15.5) | 35 (16.9) | 5 (9.8) | |
| 5%–25 % | 80 (31.0) | 59 (28.5) | 21 (41.2) | |
| 25%–50 % | 32 (12.4) | 24 (11.6) | 8 (15.7) | |
| 50%–75 % | 43 (16.7) | 31 (15.0) | 12 (23.5) | |
| 75%–100 % | 49 (19.0) | 44 (21.3) | 5 (9.8) |
Data are presented as mean ± standard deviation (SD), medians (IQR) and n (%). P values were calculated by Student t-test, Mann–Whitney U test, Chi-square test or Fisher's exact test, as appropriate. P values indicate differences between COPD and CPFE.
3.2. Clinical characteristics and laboratory findings of the study population
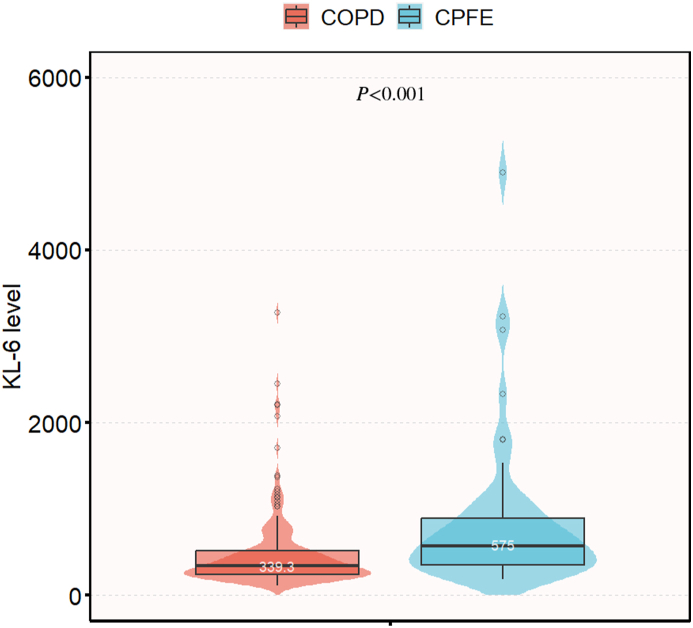
The most common clinical symptoms reported were cough (87.2 %), expectoration (82.6 %), and dyspnea (68.2 %). In terms of lung function, CPFE patients exhibited relatively preserved pulmonary function, including higher values of forced expiratory volume in 1 s (FEV1) (1.97 vs 1.57, P = 0.002), FEV1% (74.24 vs 62.99, P = 0.012), and FEV1/forced vital capacity (FEV1/FVC) ratio (69.46 vs 57.64, P < 0.001) compared to COPD patients. Thoracic CT scans, which were accessible for all patients, detected the presence of emphysema in 245 cases, while the remaining 14 cases were diagnosed with COPD of the chronic bronchitis type. The distribution of emphysema extent was as follows: 0 % (5.4 %), <5 % (15.5 %), 5%–25 % (31.0 %), 25%–50 % (12.4 %), 50%–75 % (16.7 %), and 75%–100 % (19.0 %) (Table 1). CPFE patients exhibited elevated eosinophil counts (0.20 vs 0.10 × 109/L, P = 0.033), percentage of eosinophils (2.10 vs 1.40 %, P = 0.028), lactate dehydrogenase (226.50 vs 206.65 U/L, P = 0.044), total bilirubin levels (12.00 vs 9.75 μmol/L, P = 0.045), and KL-6 levels (574.95 vs 339.30 U/mL, P < 0.001; Fig. 1) compared to COPD patients. Conversely, COPD patients demonstrated higher levels of platelet counts (215.00 vs 185.50 × 109/L, P = 0.025) compared to CPFE patients, with both groups' values falling within the normal range. Both groups exhibited elevated levels of C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), Interleukin 6 (IL-6) and Tumor necrosis factor α (TNF-α) beyond the normal range; however, there was no significant difference observed between the COPD and CPFE groups (Table 2).
Fig. 1.
Divergence in KL-6 levels between the COPD group and the CPFE group.
Table 2.
Laboratory findings at admission between subjects with COPD and CPFE.
| Laboratory findings | Normal Range | Total (n = 259) | COPD (n = 207) | CPFE (n = 52) | P Value |
|---|---|---|---|---|---|
| Blood Routine | |||||
| Red blood cell count, × 1012/L | 3.80–5.10 | 3.97 (3.54–4.40) | 3.97 (3.56–4.40) | 3.91 (3.44–4.44) | 0.595 |
| Hemoglobin, g/L | 130–175 | 119.00 (103.00–131.00) | 119.00 (103.00–131.00) | 119.50 (106.00–132.00) | 0.646 |
| White blood cell count, × 109/L | 3.5–9.5 | 7.40 (5.60–9.70) | 7.60 (5.70–9.70) | 6.85 (4.85–8.85) | 0.168 |
| Neutrophil count, × 109/L | 1.8–6.3 | 5.30 (3.50–7.40) | 5.40 (3.60–7.50) | 4.70 (3.03–6.98) | 0.245 |
| Neut% | 40.0–75.0 | 71.40 (61.80–82.60) | 72.40 (62.20–83.10) | 68.25 (59.93–91.35) | 0.582 |
| Lymphocyte count, × 109/L | 1.1–3.2 | 1.20 (0.70–1.60) | 1.20 (0.70–1.60) | 1.10 (0.80–1.48) | 0.595 |
| Lymph% | 20.0–50.0 | 16.80 (9.00–24.90) | 16.60 (8.50–24.90) | 17.45 (10.95–25.38) | 0.522 |
| Eosinophil count, × 109/L | 0.02–0.52 | 0.10 (0.05–0.20) | 0.10 (0.04–0.20) | 0.20 (0.08–0.30) | 0.033 |
| Eos% | 0.4–8.0 | 1.40 (0.60–3.40) | 1.40 (0.50–3.00) | 2.10 (0.90–5.00) | 0.028 |
| Platelet count, × 109/L |
125–350 |
208.00 (158.00–273.00) |
215.00 (165.00–279.00) |
185.50 (136.50–232.00) |
0.025 |
| Blood Biochemistry | |||||
| Glucose, mmol/L | 3.9–5.8 | 5.50 (4.73–7.45) | 5.50 (4.78–7.45) | 5.60 (4.47–7.61) | 0.597 |
| Aspartate aminotransferase, U/L | 15.0–40.0 | 23.90 (18.60–32.15) | 23.85 (18.60–31.25) | 24.90 (18.20–34.60) | 0.632 |
| Alanine aminotransferase, U/L | 9.0–50.0 | 17.70 (11.85–29.25) | 17.85 (12.05–28.50) | 16.70 (11.50–35.80) | 0.640 |
| Lactate dehydrogenase, U/L | 120.0–250.0 | 210.00 (172.25–271.55) | 206.65 (169.63–266.50) | 226.50 (183.00–295.00) | 0.044 |
| Total bile acid, μmol/L | 0–12.0 | 3.80 (2.20–6.00) | 3.60 (2.00–5.90) | 4.10 (2.50–7.80) | 0.125 |
| Total bilirubin, μmol/L | 0–25.0 | 10.20 (7.40–14.40) | 9.75 (7.10–14.00) | 12.00 (8.50–14.50) | 0.045 |
| Albumin, g/L | 40.0–55.0 | 34.50 (30.50–38.50) | 34.50 (30.65–38.53) | 34.30 (30.40–38.50) | 0.742 |
| Blood uric acid, μmol/L | 208.0–428.0 | 315.89 ± 112.98 | 315.22 ± 116.61 | 318.55 ± 97.95 | 0.851 |
| Blood urea, mmol/L | 3.60–9.50 | 6.02 (4.63–7.66) | 6.03 (4.69–7.83) | 6.01 (4.28–7.54) | 0.620 |
| Serum creatinine, μmol/L | 41.0–111.0 | 77.00 (64.25–90.00) | 76.90 (64.08–90.78) | 77.20 (65.00–87.00) | 0.942 |
| C-reactive protein, mg/L | 0–8.00 | 21.70 (7.57–78.03) | 18.95 (7.68–77.75) | 28.00 (6.11–94.70) | 0.621 |
| Erythrocyte sedimentation rate, mm/h | 0–21 | 59.50 (35.00–88.00) | 60.00 (32.00–88.00) | 56.00 (41.00–104.00) | 0.105 |
| Complement C3, mg/L | 790.00–1520.00 | 922.44 ± 221.31 | 934.39 ± 220.30 | 877.96 ± 221.94 | 0.138 |
| Complement C4, mg/L | 100.00–400.00 | 254.14 ± 79.07 | 256.15 ± 77.74 | 246.61 ± 84.38 | 0.483 |
| Interleukin 6, pg/mL | <5.9 | 10.90 (5.08–28.23) | 11.30 (5.11–27.28) | 10.08 (4.30–35.35) | 0.917 |
| Tumor necrosis factor α, pg/mL | <8.1 | 10.70 (7.79–14.38) | 10.50 (7.67–13.70) | 11.50 (8.06–17.40) | 0.207 |
| Krebs von den Lungen-6, U/mL |
105.3–401.2 |
364.60 (253.10–606.80) |
339.30 (239.70–514.40) |
574.95 (344.48–941.60) |
<0.001 |
| Myocardial Injury Mediators | |||||
| Creatine kinase, U/L | 50.0–310.0 | 50.40 (31.98–77.83) | 52.85 (33.48–77.08) | 44.90 (27.00–92.35) | 0.495 |
| Myoglobin, μg/mL | <70 | 38.10 (26.63–55.90) | 36.45 (25.73–55.90) | 43.30 (31.23–55.98) | 0.214 |
| Creatine kinase-MB, U/L | <24.0 | 12.20 (9.20–16.25) | 12.30 (9.13–16.33) | 12.05 (9.30–16.33) | 0.871 |
| N-Terminal pro-brain natriuretic peptide (NT-proBNP), pg/mL |
0–450 |
309.49 (103.95–824.46) |
311.00 (119.00–861.26) |
287.67 (94.54–634.82) |
0.799 |
| Blood Coagulation | |||||
| D-dimer, μg/mL | 0–0.5 | 0.32 (0.16–0.85) | 0.31 (0.16–0.85) | 0.39 (0.16–0.88) | 0.706 |
| Prothrombin time (PT), s | 9.0–14.0 | 11.75 (11.00–12.60) | 11.70 (11.00–12.60) | 12.00 (11.00–13.20) | 0.123 |
| Activated partial thromboplastin time (APTT), s | 22.3–32.5 | 28.25 (26.50–31.68) | 28.15 (26.20–31.73) | 29.65 (26.88–31.60) | 0.229 |
| Prothrombin Time - International Normalized Ratio (PT-INR) |
0.8–1.2 |
0.99 (0.92–1.09) |
0.99 (0.92–1.09) |
1.02 (0.94–1.14) |
0.063 |
| Blood lipid | |||||
| Triglyceride, mmol/L | <1.70 | 1.14 (0.88–1.58) | 1.17 (0.88–1.60) | 1.12 (0.88–1.50) | 0.613 |
| Low density lipoprotein cholesterol, mmol/L | 1.55–3.19 | 2.66 (2.09–3.28) | 2.71 (2.09–3.33) | 2.55 (2.15–3.03) | 0.174 |
| Total cholesterol, mmol/L | <5.18 | 4.11 (3.33–4.99) | 4.22 (3.33–5.22) | 3.85 (3.32–4.56) | 0.136 |
| High density lipoprotein cholesterol, mmol/L | 1.04–1.55 | 0.94 (0.76–1.17) | 0.97 (0.78–1.21) | 0.86 (0.68–1.13) | 0.051 |
Data are presented as mean ± standard deviation (SD), medians (IQR) and n (%). P values were calculated by Student t-test, Mann–Whitney U test, as appropriate. P values indicate differences of characteristics between subjects with COPD and CPFE.
3.3. The imaging features of CPFE
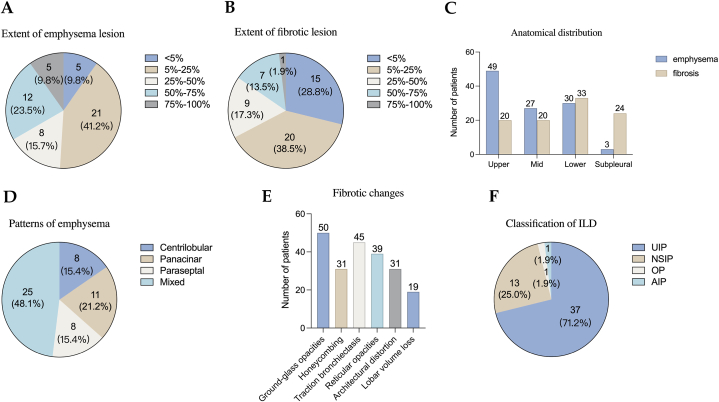
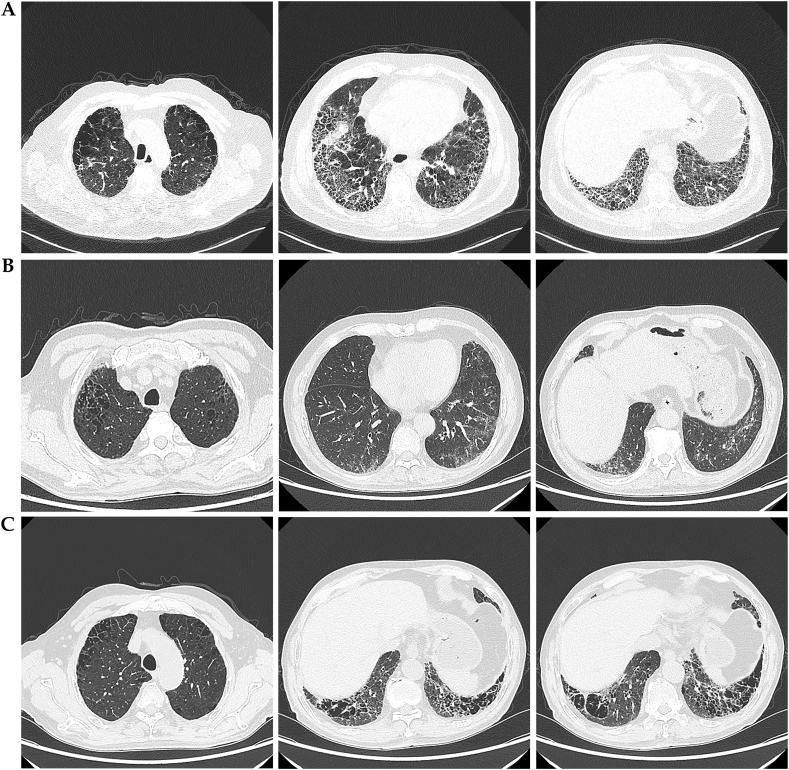
According to the predefined inclusion criteria, the chest CT scans of 52 patients demonstrated the coexistence of emphysema and pulmonary fibrosis (Supplementary Table 2). The extent of emphysema and fibrotic lesions is summarized in Fig. 2A and B. The distribution of emphysema predominantly affected the upper lobes (94.2 %), while the fibrosis primarily localized to the lower lobes (63.5 %) (Fig. 2C). Regarding the classification of emphysema types, 25 patients (48.1 %) were categorized as mixed type, 8 patients (15.4 %) as centrilobular type, 11 patients (21.2 %) as panacinar type, and 8 patients (15.4 %) as paraseptal type (Fig. 2D). Ground-glass opacities, traction bronchiectasis, and reticular opacities were the most frequently observed findings, present in 96.2 %, 86.5 %, and 75.0 % of the cases, respectively (Fig. 2E). Based on high-resolution CT (HRCT) evaluations, the diagnoses were classified as usual interstitial pneumonia (UIP) in 37 patients (71.2 %) (Fig. 3A), nonspecific interstitial pneumonia (NSIP) in 13 patients (25.0 %) (Fig. 3B), and neither UIP nor NSIP in the remaining cases (Fig. 2F). Thick-walled large cysts, representing a distinctive imaging pattern of CPFE, were observed in 14 (26.9 %) of the patients (Fig. 3C and Supplementary Fig. 2).
Fig. 2.
The imaging features of CPFE. (A) Extent of emphysema lesion. (B) Extent of fibrotic lesion. (C) The anatomical distribution of emphysema and fibrosis. (D) Patterns of emphysema. (E) Fibrotic changes. (F) Classification of ILD.
Fig. 3.
High-resolution computed tomography showing a typical distribution of disease seen in combined pulmonary fibrosis and emphysema. (A) HRCT findings of a 75-year-old male with CPFE. Bilateral upper lung lobes exhibit central and paraseptal emphysema. Interstitial lesions in the right middle lobe, left upper lobe lingular segment, and bilateral lower lungs present as honeycombing. Traction bronchiectasis and decreased lung volumes are observed. Interstitial lesion pattern consistent with UIP. (B) HRCT findings of a 69-year-old male with CPFE. Bilateral upper lung lobes exhibit centrilobular and panlobular emphysema. Interstitial lesions in bilateral lower lungs appear as ground-glass opacities without honeycombing or traction bronchiectasis. Interstitial lesion pattern consistent with NSIP. (C) HRCT findings of a 69-year-old male with CPFE demonstrate admixed emphysema and fibrosis with thick-walled large cysts. There is central and peribronchiolar emphysema in the upper lung lobes, while the lower lobes exhibit interstitial lung disease characterized by honeycomb opacities accompanied by thick-walled large cysts.
3.4. Serum KL-6 levels are associated with the presence of CPFE
To enhance the clinical value of our data, we have rescaled the KL-6 values by dividing them by 100. Through logistic regression analysis, we investigated factors associated with CPFE and identified rheumatic diseases, platelet count, international normalized ratio (INR), high density lipoprotein cholesterol (HDL-C) and KL-6 as potential indicators of CPFE. Further analysis using multivariable regression demonstrated that KL-6 levels were independently associated with CPFE. Specifically, for every 100 U/mL increase in KL-6, the likelihood of CPFE incidence increased by 1.11 times (OR 1.11, 95 % CI 1.04–1.17, P = 0.001; Table 3). To assess the robustness of our adjusted model, we performed stratified analyses based on age, gender, rheumatic diseases, platelet count, INR and HDL-C. The forest plot revealed no significant interactions among these subgroups (P > 0.05, Fig. 4).
Table 3.
Factors associated with CPFE.
| Variables | OR (95%CI) | P value | Adjusted OR (95%CI) | P value |
|---|---|---|---|---|
| Age | 1.01 (0.97–1.05) | 0.693 | ||
| Gender (male) | 2.97 (0.68–13.07) | 0.149 | ||
| Smoking status (current smoker) | 3.52 (0.78–15.93) | 0.103 | ||
| Smoking status (former smoker) | 3.57 (0.79–16.08) | 0.097 | ||
| Pack years |
1.00 (0.99–1.01) |
0.919 |
||
| Comorbidities | ||||
| Lung cancer | 0.61 (0.33–1.15) | 0.127 | ||
| Rheumatic diseases | 3.43 (1.47–8.02) | 0.004 | 4.13 (1.22–13.94) | 0.022 |
| Pulmonary hypertension | 0.66 (0.29–1.49) | 0.313 | ||
| Hypertension | 0.93 (0.50–1.75) | 0.829 | ||
| Coronary heart disease | 0.85 (0.42–1.74) | 0.656 | ||
| Cerebrovascular diseases | 1.29 (0.49–3.41) | 0.607 | ||
| Diabetes | 1.54 (0.76–3.11) | 0.230 | ||
| Tuberculosis | 0.81 (0.41–1.59) | 0.531 | ||
| Chronic liver diseases |
0.68 (0.19–2.43) |
0.557 |
||
| Blood test | ||||
| Hb | 1.01 (0.99–1.02) | 0.506 | ||
| Neut | 0.95 (0.86–1.05) | 0.295 | ||
| Lymph | 0.82 (0.51–1.32) | 0.419 | ||
| Eos | 2.83 (0.57–14.16) | 0.205 | ||
| Plt | 1.00 (0.99–1.00) | 0.043 | 1.00 (0.99–1.00) | 0.064 |
| ALB | 0.98 (0.93–1.04) | 0.544 | ||
| LDH | 1.00 (1.00–1.00) | 0.436 | ||
| TBIL | 1.01 (0.98–1.05) | 0.459 | ||
| INR | 10.27 (1.65–64.07) | 0.013 | 4.18 (0.43–40.38) | 0.216 |
| CRP | 1.00 (1.00–1.00) | 0.387 | ||
| ESR | 1.01 (1.00–1.02) | 0.102 | ||
| C3 | 1.00 (1.00–1.00) | 0.139 | ||
| C4 | 1.00 (0.99–1.00) | 0.482 | ||
| TC | 0.82 (0.64–1.06) | 0.137 | ||
| HDL-C | 0.35 (0.13–0.98) | 0.045 | 0.42 (0.12–1.44) | 0.582 |
| KL-6/100 (U/mL) | 1.11 (1.05–1.17) | <0.001 | 1.11 (1.04–1.17) | 0.001 |
Abbreviations: Hb, hemoglobulin; Neut, neutrophil; Lymph, Lymphocyte; Eos, eosinophil; PLT, platelet; ALB, Albumin; LDH, Lactate dehydrogenase; TBIL, Total bilirubin; INR, International normalized ratio; CRP, C-reaction protein; ESR, erythrocyte sedimentation rate; C3, complement C3; C4, complement C4; TC, total cholesterol; HDL-C, High density lipoprotein cholesterol; KL-6, Krebs Von den Lungen-6.
Fig. 4.
Forest plot for the subgroup analysis of the presence of CPFE according to KL-6 levels. For each group of interest, the gray horizontal lines represent the 95 % confidence interval (CI).
3.5. The role of KL-6 in distinguishing CPFE from COPD
To evaluate the diagnostic utility of serum KL-6 in distinguishing CPFE from COPD in clinical settings, we conducted receiver operating characteristic curve (ROC) analysis. The area under the curve (AUC) was calculated as 0.711, with a 95 % confidence interval (CI) of 0.635–0.787 (P < 0.001). The optimal cut-off point of KL-6 was possibly determined to be around 550.95 U/mL based on the maximum Youden index, indicating its potential value in discriminating CPFE among COPD patients. At this threshold, the corresponding sensitivity was found to be 57.7 %, while the specificity was 79.7 % (Fig. 5).
Fig. 5.
ROC curve of KL-6 to differentiate CPFE from COPD.
4. Discussion
In this study, we conducted an initial statistical analysis of demographic data and selected biomarkers, which revealed significant associations between CPFE and various indicators. Subsequently, rheumatic diseases, platelet count, INR, HDL-C and KL-6 were subjected to multivariate logistic regression analysis to assess their predictive relevance for distinguishing between CPFE and COPD. The results demonstrated that rheumatic diseases and KL-6 exhibited statistical significance (P < 0.05). Furthermore, we employed ROC curve analysis to evaluate the diagnostic efficacy of KL-6. Our findings indicated that KL-6 could potentially serve as a biomarker for differentiating CPFE from COPD. The AUC was calculated as 0.711, with a 95 % CI of 0.635–0.787. Based on the maximum Youden index, the optimal cut-off point of KL-6 was estimated to be around 550.95 U/mL, with a sensitivity of 57.7 % and specificity of 79.7 %. Consequently, a KL-6 level higher than 550.95 U/mL in COPD patients suggests the potential requirement for HRCT to identify the presence of fibrosis.
KL-6 serves as a valuable serum biomarker for diagnosing various types of ILD and is closely associated with disease activity [14,15]. Additionally, elevated serum KL-6 levels are also associated with acute exacerbation and mortality in cases of CPFE [16]. In this study, we further validate that KL-6 can effectively differentiate individuals with CPFE within the COPD population. Therefore, in the presence of elevated KL-6 levels in patients with COPD, it is imperative for clinicians to perform HRCT to assess the potential presence of fibrosis. Furthermore, the identification of a critical value of 550.95 U/mL in the CPFE group, which surpasses the established cutoff value of 500 U/mL for interstitial pneumonia [9], potentially suggests a heightened severity of injury to bronchial epithelial cells or type II alveolar epithelial cells in CPFE when compared to cases of interstitial pneumonia.
In our study, platelet count exhibited a significant association with CPFE in the univariate regression analysis. This finding aligns with a systematic review that reported a significant increase in platelet count among individuals with COPD when compared to non-COPD controls [17], while another study demonstrated a statistically significant decrease in mean platelet count among patients with IPF in comparison to control subjects [18].
In the analysis of HRCT images, the coexistence of emphysema and interstitial fibrosis characterizes CPFE, resulting in a diverse range of manifestations. In our study involving 52 patients with CPFE, we observed that the distribution of emphysema was predominantly characterized by a mixed pattern, which aligns with findings from previous research [19]. Notably, the emphysema exhibited a predominant distribution within the upper lobes, whereas the fibrosis primarily localized to the lower lobes. Additionally, several cases showed concurrent spatial involvement of both emphysema and fibrosis. The association between fibrosis and emphysema exhibited variability, consistent with previous findings [20]. Ground-glass opacities, traction bronchiectasis, and reticular opacities were the most frequent findings. As previously mentioned, UIP is the most frequently identified pattern in ILD [21]. However, other types of ILD, such as NSIP, OP, and AIP have also been reported. Previous studies have indicated that admixed emphysema and thick-walled large cysts may represent characteristics of CPFE [22,23]. In our study, thick-walled large cysts were observed in 26.9 % of the patients, which closely aligns with the previously reported prevalence of 29 % [22]. This finding suggests that our study potentially exhibits a promising level of representativeness in reflecting the characteristics of CPFE.
There were notable differences in lung function test results between patients with CPFE and those with COPD. As previously reported [24,25], parameters such as FEV1, percent predicted FEV1, and FEV1/FVC showed significant variations, with higher values observed in the CPFE group compared to the COPD group. Additionally, a cohort study demonstrated a positive correlation between serum bilirubin levels and FEV1, FVC, and FEF25–75 % [25], which further supports our conclusion that CPFE patients exhibit higher TBIL levels compared to COPD patients. Notably, our study revealed that although the CPFE group exhibited lower DLCO, there was no statistically significant difference in DLCO between the COPD and CPFE groups. Previous studies have reported a substantial decrease in DLCO among CPFE patients [26], with the extent of fibrosis exerting a more pronounced impact on DLCO than emphysema [20]. These findings may suggest a relatively higher proportion of patients with mild disease in our study population, as confirmed by the extent of fibrosis observed in the HRCT scans.
We have observed a significant correlation between CPFE syndrome and rheumatic diseases. This observation is consistent with several independent reports that have suggested the potential role of connective tissue diseases as a risk factor for the development of emphysema, regardless of smoking status [[27], [28], [29], [30]]. Therefore, our findings provide further support for the notion that rheumatic diseases may indeed contribute significantly to the pathogenesis of CPFE. Considering the diverse nature of rheumatic diseases and their associated lesions, we conducted analysis based on disease classification. However, due to the sample size limitations, statistical differences were not observed between the groups. Conversely, previous studies consistently reported a higher incidence of pulmonary hypertension and lung cancer in the CPFE group compared to the emphysema group [1,24,31,32]. It is important to acknowledge that the divergent findings in our study may be influenced by potential biases in patient selection and limitations arising from a relatively small sample size. However, both the student t-test and multivariate logistic regression analysis did not reveal a significant difference in the prevalence of pulmonary hypertension and lung cancer between the CPFE and COPD groups. Additionally, the forest plot generated from stratified analyses indicated no significant interactions among the subgroups of rheumatic diseases. Based on these results, we can infer that even when considering the potential presence of selection bias, the presence of comorbidities does not impact the diagnostic value of KL-6 in identifying CPFE within the COPD population after appropriate adjustment for confounding factors.
The present study has several notable limitations that warrant acknowledgment. Firstly, it is important to recognize that this study is retrospective in nature. However, this retrospective design does not undermine the main findings of our study, which suggest that the KL-6 test may serve as a practical tool for differentiating CPFE from COPD. Secondly, as a single-center study, our findings may not fully capture the diverse spectrum of patients treated at local primary or secondary care centers. Nonetheless, our study likely provides insights into typical or real-world scenarios. Furthermore, we recognize that our dataset has missing values, particularly in relation to inadequate blood type data, however, it is crucial to emphasize that this parameter is not the principal research measures in our study, as such, it has no impact on the validity or interpretation of our main findings. Additionally, the limited sample size imposes constraints on our ability to accurately validate the calculated AUC. Thus, future research endeavors should prioritize larger prospective multicenter cohort studies to robustly validate our observations.
5. Conclusions
Our study findings indicate that patients with CPFE exhibit significantly higher KL-6 levels compared to those with isolated COPD. This suggests that KL-6 has the potential to serve as a practical screening tool for interstitial lung disease, specifically CPFE. Furthermore, a KL-6 threshold of 550.95 U/mL in COPD patients may indicate the necessity of high-resolution chest computed tomography for the detection of fibrosis.
Fundings
This study was supported by grants from The Youth Science Foundation of Xiangya Hospital (2022Q06 to Dr. Aiyuan Zhou), the Natural Science Foundation of Hunan Province, China (Grant No.2023JJ41025 to Dr. Aiyuan Zhou), the Scientific Research Project of Hunan Health Commission (Grant No.D202303029041), Project Program of National Clinical Research Center for Geriatric Disorders (Xiangya Hospital, Grant No. 2020LNJJ05), The National Key Clinical Specialist Construction Program of China (Grant Number z047-02), Project Program of central south university graduate education teaching reform (No.2022JGB025), The Scientific Research Program of FuRong Laboratory (No.2023SK2101), and Key R & D Program of Hunan Province (No.2022SK2038).
Ethical approval and consent to participate
This study was reviewed and approved by the local Ethics Committee of the Xiangya Hospital of Central South University with the approval number: No. 202309183, dated September 6th, 2023.
Data availability
Data will be made available on request.
CRediT authorship contribution statement
Aiyuan Zhou: Writing – original draft, Investigation, Formal analysis, Data curation. Xiyan Zhang: Writing – original draft, Investigation, Formal analysis, Data curation. Rongli Lu: Software, Investigation, Formal analysis. Wenzhong Peng: Investigation, Resources, Validation. Yanan Wang: Writing – review & editing, Resources. Haiyun Tang: Visualization, Supervision, Funding acquisition, Conceptualization. Pinhua Pan: Project administration, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We gratefully thank the Physician-Scientist Team for their contributions to the statistical support, study design consultations, and comments regarding the manuscript. We acknowledge the professionalism and compassion demonstrated by all the healthcare workers involved in patient care. The authors thank all study participants for their involvement in this study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e35099.
Contributor Information
Haiyun Tang, Email: 405016@csu.edu.cn.
Pinhua Pan, Email: pinhuapan668@csu.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Cottin V., et al. Syndrome of combined pulmonary fibrosis and emphysema: an official ATS/ERS/JRS/ALAT research statement. Am. J. Respir. Crit. Care Med. 2022;206(4):e7–e41. doi: 10.1164/rccm.202206-1041ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee C.H., et al. The impact of combined pulmonary fibrosis and emphysema on mortality. Int. J. Tubercul. Lung Dis. 2011;15(8):1111–1116. doi: 10.5588/ijtld.10.0491. [DOI] [PubMed] [Google Scholar]
- 3.Hage R., et al. Combined pulmonary fibrosis and emphysema (CPFE) clinical features and management. Int. J. Chronic Obstr. Pulm. Dis. 2021;16:167–177. doi: 10.2147/COPD.S286360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nasim F., Moua T. Lung cancer in combined pulmonary fibrosis and emphysema: a large retrospective cohort analysis. ERJ Open Res. 2020;6(4) doi: 10.1183/23120541.00521-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robledo G.C., et al. Combined pulmonary fibrosis and emphysema with pulmonary hypertension: cases report. Curr. Probl. Cardiol. 2022;47(4) doi: 10.1016/j.cpcardiol.2021.100856. [DOI] [PubMed] [Google Scholar]
- 6.Hirasawa Y., et al. KL-6, a human MUC1 mucin, is chemotactic for human fibroblasts. Am. J. Respir. Cell Mol. Biol. 1997;17(4):501–507. doi: 10.1165/ajrcmb.17.4.2253. [DOI] [PubMed] [Google Scholar]
- 7.Lee J.S., et al. Serum KL-6 levels reflect the severity of interstitial lung disease associated with connective tissue disease. Arthritis Res. Ther. 2019;21(1):58. doi: 10.1186/s13075-019-1835-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou A., et al. KL-6 levels in the connective tissue disease population: typical values and potential confounders-a retrospective, real-world study. Front. Immunol. 2023;14 doi: 10.3389/fimmu.2023.1098602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kobayashi J., et al. [Establishment of reference intervals and cut-off value by an enzyme immunoassay for KL-6 antigen, a new marker for interstitial pneumonia] Rinsho Byori. 1996;44(7):653–658. [PubMed] [Google Scholar]
- 10.Higham A., et al. The pathology of small airways disease in COPD: historical aspects and future directions. Respir. Res. 2019;20(1):49. doi: 10.1186/s12931-019-1017-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller M.R., et al. Standardisation of spirometry. Eur. Respir. J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 12.Quanjer P.H., et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur. Respir. J. 2012;40(6):1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu L. [The controversies and management strategies in diagnosis of pulmonary function] Zhonghua Jiehe He Huxi Zazhi. 2015;38(6):405–407. [PubMed] [Google Scholar]
- 14.Kohno N., et al. New serum indicator of interstitial pneumonitis activity. Sialylated carbohydrate antigen KL-6. Chest. 1989;96(1):68–73. doi: 10.1378/chest.96.1.68. [DOI] [PubMed] [Google Scholar]
- 15.Ohnishi H., et al. Comparative study of KL-6, surfactant protein-A, surfactant protein-D, and monocyte chemoattractant protein-1 as serum markers for interstitial lung diseases. Am. J. Respir. Crit. Care Med. 2002;165(3):378–381. doi: 10.1164/ajrccm.165.3.2107134. [DOI] [PubMed] [Google Scholar]
- 16.Kishaba T., et al. A cohort study of mortality predictors and characteristics of patients with combined pulmonary fibrosis and emphysema. BMJ Open. 2012;2(3) doi: 10.1136/bmjopen-2012-000988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zinellu A., et al. Platelet count and platelet indices in patients with stable and acute exacerbation of chronic obstructive pulmonary disease: a systematic review and meta-analysis. COPD. 2021;18(2):231–245. doi: 10.1080/15412555.2021.1898578. [DOI] [PubMed] [Google Scholar]
- 18.Ntolios P., et al. Mean platelet volume as a surrogate marker for platelet activation in patients with idiopathic pulmonary fibrosis. Clin. Appl. Thromb. Hemost. 2016;22(4):346–350. doi: 10.1177/1076029615618023. [DOI] [PubMed] [Google Scholar]
- 19.Alsumrain M., et al. Combined pulmonary fibrosis and emphysema as a clinicoradiologic entity: characterization of presenting lung fibrosis and implications for survival. Respir. Med. 2019;146:106–112. doi: 10.1016/j.rmed.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 20.Cottin V., et al. Combined pulmonary fibrosis and emphysema: a distinct underrecognised entity. Eur. Respir. J. 2005;26(4):586–593. doi: 10.1183/09031936.05.00021005. [DOI] [PubMed] [Google Scholar]
- 21.Ciccarese F., Attinà D., Zompatori M. Combined pulmonary fibrosis and emphysema (CPFE): what radiologist should know. Radiol. Med. 2016;121(7):564–572. doi: 10.1007/s11547-016-0627-4. [DOI] [PubMed] [Google Scholar]
- 22.Cottin V., et al. Combined pulmonary fibrosis and emphysema syndrome in connective tissue disease. Arthritis Rheum. 2011;63(1):295–304. doi: 10.1002/art.30077. [DOI] [PubMed] [Google Scholar]
- 23.Inomata M., et al. An autopsy study of combined pulmonary fibrosis and emphysema: correlations among clinical, radiological, and pathological features. BMC Pulm. Med. 2014;14:104. doi: 10.1186/1471-2466-14-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kitaguchi Y., et al. Clinical characteristics of combined pulmonary fibrosis and emphysema. Respirology. 2010;15(2):265–271. doi: 10.1111/j.1440-1843.2009.01676.x. [DOI] [PubMed] [Google Scholar]
- 25.Leem A.Y., et al. Association of serum bilirubin level with lung function decline: a Korean community-based cohort study. Respir. Res. 2018;19(1):99. doi: 10.1186/s12931-018-0814-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kitaguchi Y., et al. Annual changes in pulmonary function in combined pulmonary fibrosis and emphysema: over a 5-year follow-up. Respir. Med. 2013;107(12):1986–1992. doi: 10.1016/j.rmed.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 27.Antoniou K.M., et al. Combined pulmonary fibrosis and emphysema in scleroderma-related lung disease has a major confounding effect on lung physiology and screening for pulmonary hypertension. Arthritis Rheumatol. 2016;68(4):1004–1012. doi: 10.1002/art.39528. [DOI] [PubMed] [Google Scholar]
- 28.Antoniou K.M., et al. Smoking-related emphysema is associated with idiopathic pulmonary fibrosis and rheumatoid lung. Respirology. 2013;18(8):1191–1196. doi: 10.1111/resp.12154. [DOI] [PubMed] [Google Scholar]
- 29.Champtiaux N., et al. Combined pulmonary fibrosis and emphysema in systemic sclerosis: a syndrome associated with heavy morbidity and mortality. Semin. Arthritis Rheum. 2019;49(1):98–104. doi: 10.1016/j.semarthrit.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 30.Jacob J., et al. Prevalence and effects of emphysema in never-smokers with rheumatoid arthritis interstitial lung disease. EBioMedicine. 2018;28:303–310. doi: 10.1016/j.ebiom.2018.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kwak N., et al. Lung cancer risk among patients with combined pulmonary fibrosis and emphysema. Respir. Med. 2014;108(3):524–530. doi: 10.1016/j.rmed.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 32.Mejía M., et al. Idiopathic pulmonary fibrosis and emphysema: decreased survival associated with severe pulmonary arterial hypertension. Chest. 2009;136(1):10–15. doi: 10.1378/chest.08-2306. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.