Abstract
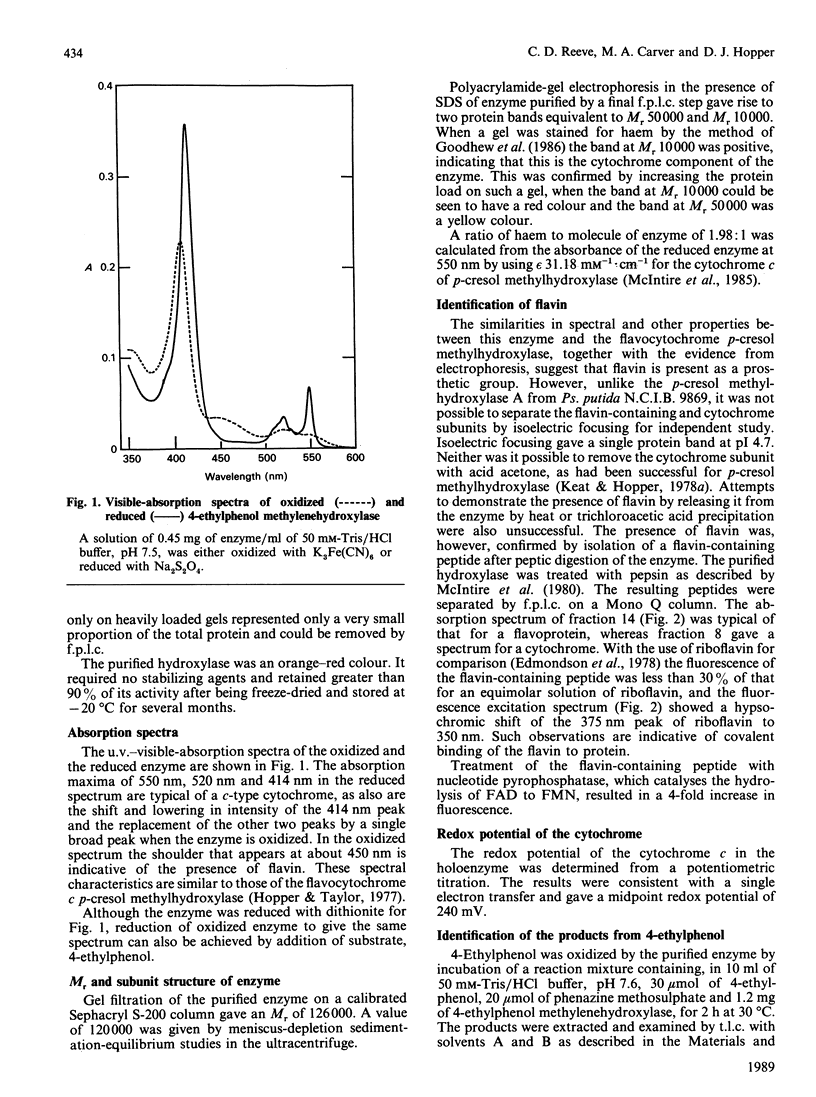
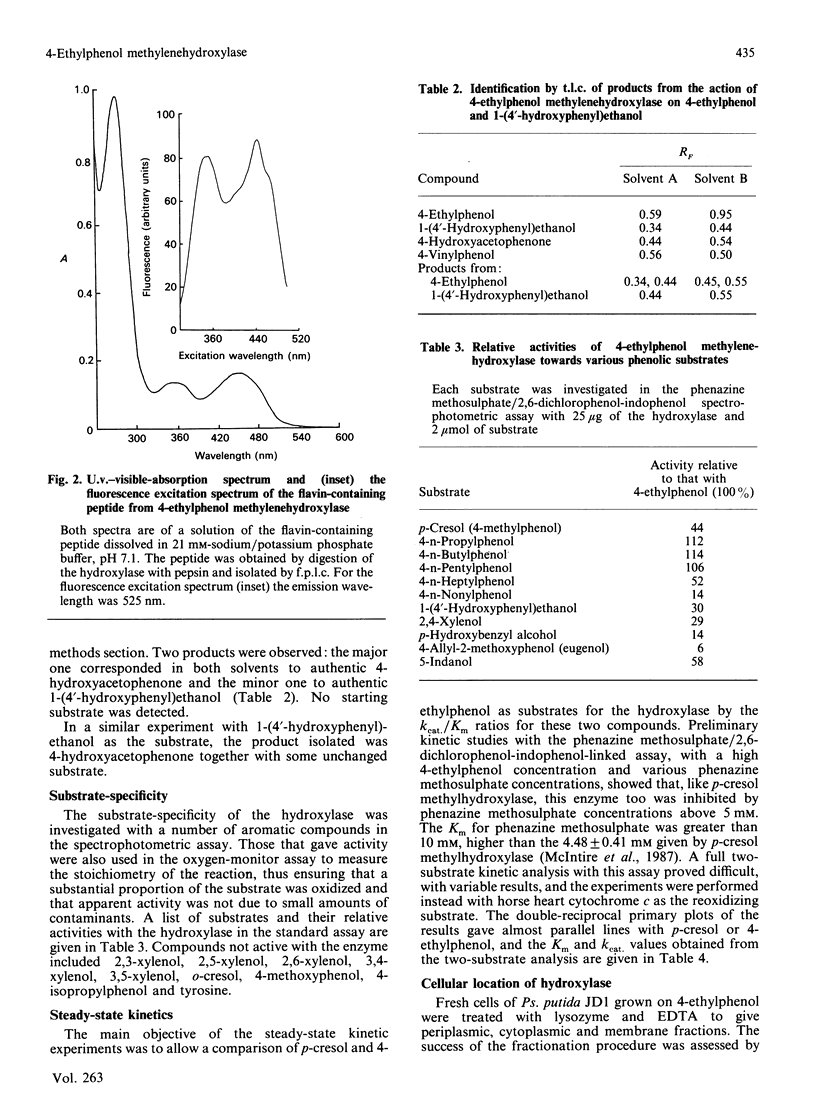
The enzyme 4-ethylphenol methylenehydroxylase was purified from Pseudomonas putida JD1 grown on 4-ethylphenol. It is a flavocytochrome c for which the Mr was found to be 120,000 by ultracentrifuging and 126,000 by gel filtration. The enzyme consists of two flavoprotein subunits each of Mr 50,000 and two cytochrome c subunits each of Mr 10,000. The redox potential of the cytochrome is 240 mV. Hydroxylation proceeds by dehydrogenation and hydration to give 1-(4'-hydroxyphenyl)ethanol, which is also dehydrogenated by the same enzyme to 4-hydroxyacetophenone. The enzyme will hydroxylate p-cresol but is more active with alkylphenols with longer-chain alkyl groups. It is located in the periplasm of the bacterium.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bossert I. D., Young L. Y. Anaerobic oxidation of p-cresol by a denitrifying bacterium. Appl Environ Microbiol. 1986 Nov;52(5):1117–1122. doi: 10.1128/aem.52.5.1117-1122.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAGLEY S., PATEL M. D. Oxidation of p-cresol and related compounds by a Pseudomonas. Biochem J. 1957 Jun;66(2):227–233. doi: 10.1042/bj0660227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton P. L. Redox potentiometry: determination of midpoint potentials of oxidation-reduction components of biological electron-transfer systems. Methods Enzymol. 1978;54:411–435. doi: 10.1016/s0076-6879(78)54026-3. [DOI] [PubMed] [Google Scholar]
- Edmondson D. E., Kenney W. C., Singer T. P. Synthesis and isolation of 8 alpha-substituted flavins and flavin peptides. Methods Enzymol. 1978;53:449–465. doi: 10.1016/s0076-6879(78)53049-8. [DOI] [PubMed] [Google Scholar]
- Hopper D. J., Jones M. R., Causer M. J. Periplasmic location of p-cresol methylhydroxylase in Pseudomonas putida. FEBS Lett. 1985 Mar 25;182(2):485–488. doi: 10.1016/0014-5793(85)80359-8. [DOI] [PubMed] [Google Scholar]
- Hopper D. J. Redox potential of the cytochrome c in the flavocytochrome p-cresol methylhydroxylase. FEBS Lett. 1983 Sep 5;161(1):100–102. doi: 10.1016/0014-5793(83)80738-8. [DOI] [PubMed] [Google Scholar]
- Hopper D. J., Taylor D. G. The purification and properties of p-cresol-(acceptor) oxidoreductase (hydroxylating), a flavocytochrome from Pseudomonas putida. Biochem J. 1977 Oct 1;167(1):155–162. doi: 10.1042/bj1670155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keat M. J., Hopper D. J. P-cresol and 3,5-xylenol methylhydroxylases in Pseudomonas putida N.C.I.B. 9896. Biochem J. 1978 Nov 1;175(2):649–658. doi: 10.1042/bj1750649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keat M. J., Hopper D. J. The aromatic alcohol dehydrogenases in Pseudomonas putida N.C.I.B. 9869 grown on 3,5-xylenol and p-cresol. Biochem J. 1978 Nov 1;175(2):659–667. doi: 10.1042/bj1750659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerber S. C., Hopper D. J., McIntire W. S., Singer T. P. Formation and properties of flavoprotein-cytochrome hybrids by recombination of subunits from different species. Biochem J. 1985 Oct 15;231(2):383–387. doi: 10.1042/bj2310383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McIntire W. S., Hopper D. J., Singer T. P. Steady-state and stopped-flow kinetic measurements of the primary deuterium isotope effect in the reaction catalyzed by p-cresol methylhydroxylase. Biochemistry. 1987 Jun 30;26(13):4107–4117. doi: 10.1021/bi00387a055. [DOI] [PubMed] [Google Scholar]
- McIntire W., Edmondson D. E., Singer T. P., Hopper D. J. 8 alpha-O-Tyrosyl-FAD: a new form of covalently bound flavin from p-cresol methylhydroxylase. J Biol Chem. 1980 Jul 25;255(14):6553–6555. [PubMed] [Google Scholar]
- McIntire W., Hopper D. J., Craig J. C., Everhart E. T., Webster R. V., Causer M. J., Singer T. P. Stereochemistry of 1-(4'-hydroxyphenyl)ethanol produced by hydroxylation of 4-ethylphenol by p-cresol methylhydroxylase. Biochem J. 1984 Dec 1;224(2):617–621. doi: 10.1042/bj2240617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntire W., Hopper D. J., Singer T. P. p-Cresol methylhydroxylase. Assay and general properties. Biochem J. 1985 Jun 1;228(2):325–335. doi: 10.1042/bj2280325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntire W., Singer T. P. Resolution of p-cresol methylhydroxylase into catalytically active subunits and reconstitution of the flavocytochrome. FEBS Lett. 1982 Jul 5;143(2):316–318. doi: 10.1016/0014-5793(82)80124-5. [DOI] [PubMed] [Google Scholar]
- McIntire W., Singer T. P., Smith A. J., Mathews F. S. Amino acid and sequence analysis of the cytochrome and flavoprotein subunits of p-cresol methylhydroxylase. Biochemistry. 1986 Oct 7;25(20):5975–5981. doi: 10.1021/bi00368a021. [DOI] [PubMed] [Google Scholar]
- ROSENBERGER R. F., ELSDEN S. R. The yields of Streptococcus faecalis grown in continuous culture. J Gen Microbiol. 1960 Jun;22:726–739. doi: 10.1099/00221287-22-3-726. [DOI] [PubMed] [Google Scholar]
- Smolenski W. J., Suflita J. M. Biodegradation of cresol isomers in anoxic aquifers. Appl Environ Microbiol. 1987 Apr;53(4):710–716. doi: 10.1128/aem.53.4.710-716.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood P. M. Why do c-type cytochromes exist? FEBS Lett. 1983 Dec 12;164(2):223–226. doi: 10.1016/0014-5793(83)80289-0. [DOI] [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]


