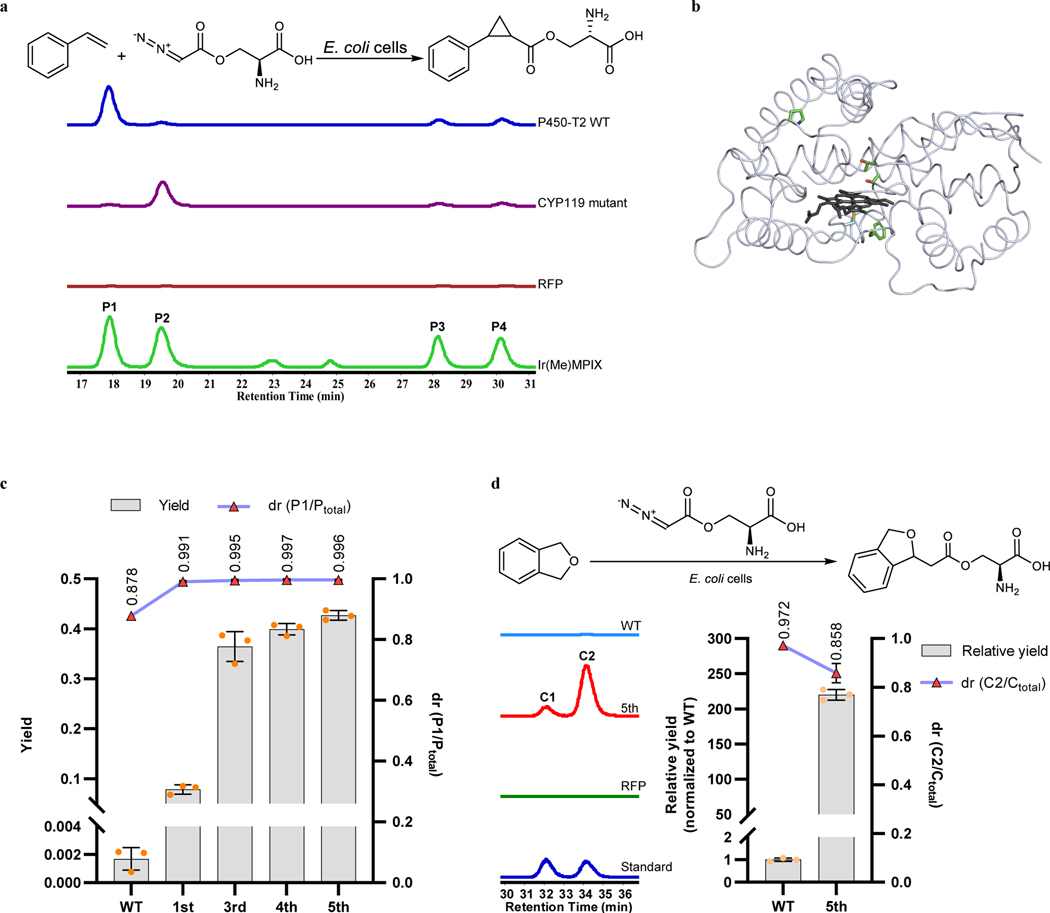
Fig. 3 |. Engineering cytochrome P450s to catalyze carbene transfer reactions with azaserine as the carbene precursor.
a, Screening P450s for catalyzing the reaction of styrene with azaserine. See the list of screened P450s and their mutants in Supplementary Table 1. EIC ([M+H]+, m/z 250.1074) for target products of reactions catalyzed by E. coli cells expressing different proteins. Representative traces are for two repeated experiments. RFP, E. coli cells expressing RFP (red fluorescence protein) as negative control; Ir(Me)MPIX, trace of the reaction products using Ir(Me)MPIX (with Na2S2O4) as catalyst for comparison. P1 to P4 are the four diastereomers formed by cyclopropanation of styrene with azaserine. b, Crystal structure of P450-T2 (PDB: 8FBC). Residues in green are mutation sites in the final evolved mutant. c, Directed evolution of P450-T2 for cyclopropanation of styrene with azaserine. d, P450-T2 WT and P450-T2–5 mutant for insertion of the carbene unit into the sp3 C–H bond of phthalan. C1 and C2 are the two diastereomers formed by the reaction of phthalan with azaserine. EIC ([M+H]+, m/z 266.1023) for target products. Representative traces for 3 biological repeats (left). Reaction conditions: 5 mM styrene or 10 mM pthalan, 5 mM azaserine, E. coli cells expressing different P450s or RFP (as control) with concentration of 30 OD600 (optical density at 600 nm) as catalysts, 5 vol% ethanol, M9-N buffer, conducted at 22 °C under aerobic conditions for 18 h. Ptotal and Ctotal, sum area for all corresponding diastereomers. In (c) and (d), numbers on the horizontal axis represent the selected mutants in each round of directed evolution (for the specific mutations, see Extended Data Table 1). Data are mean ± s.d.; n = 3 biological replicates.