Abstract
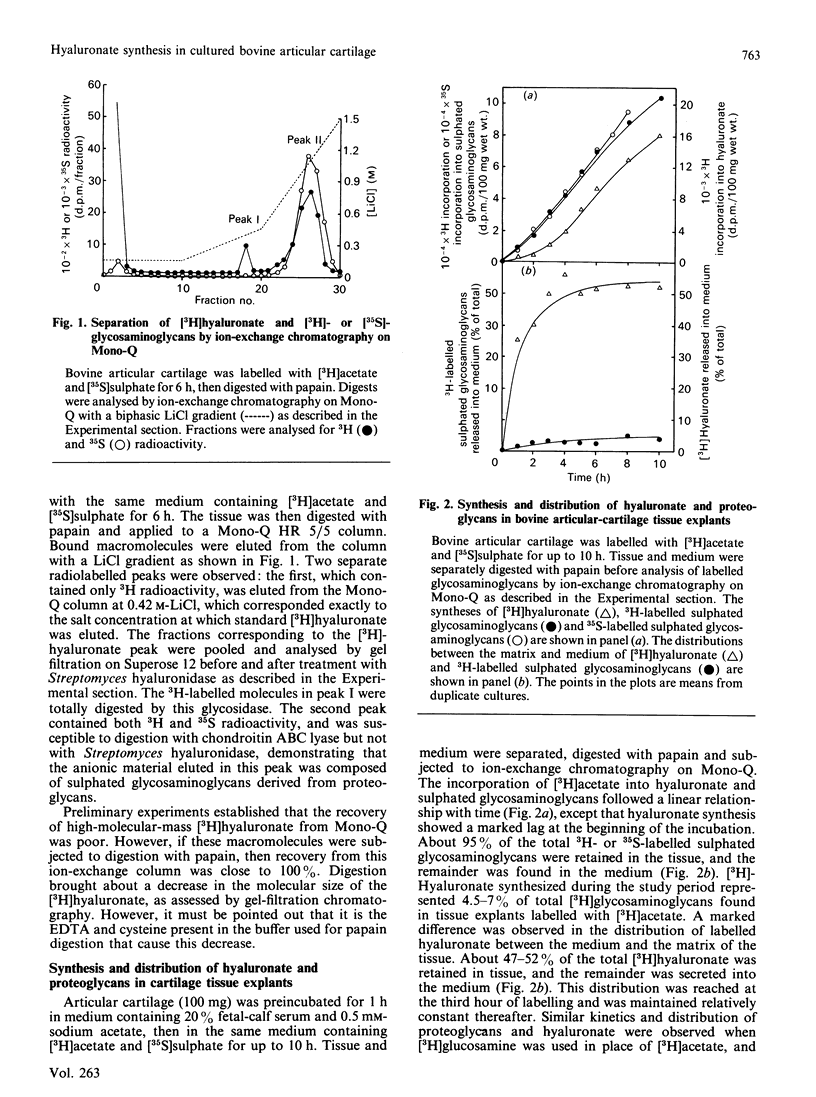
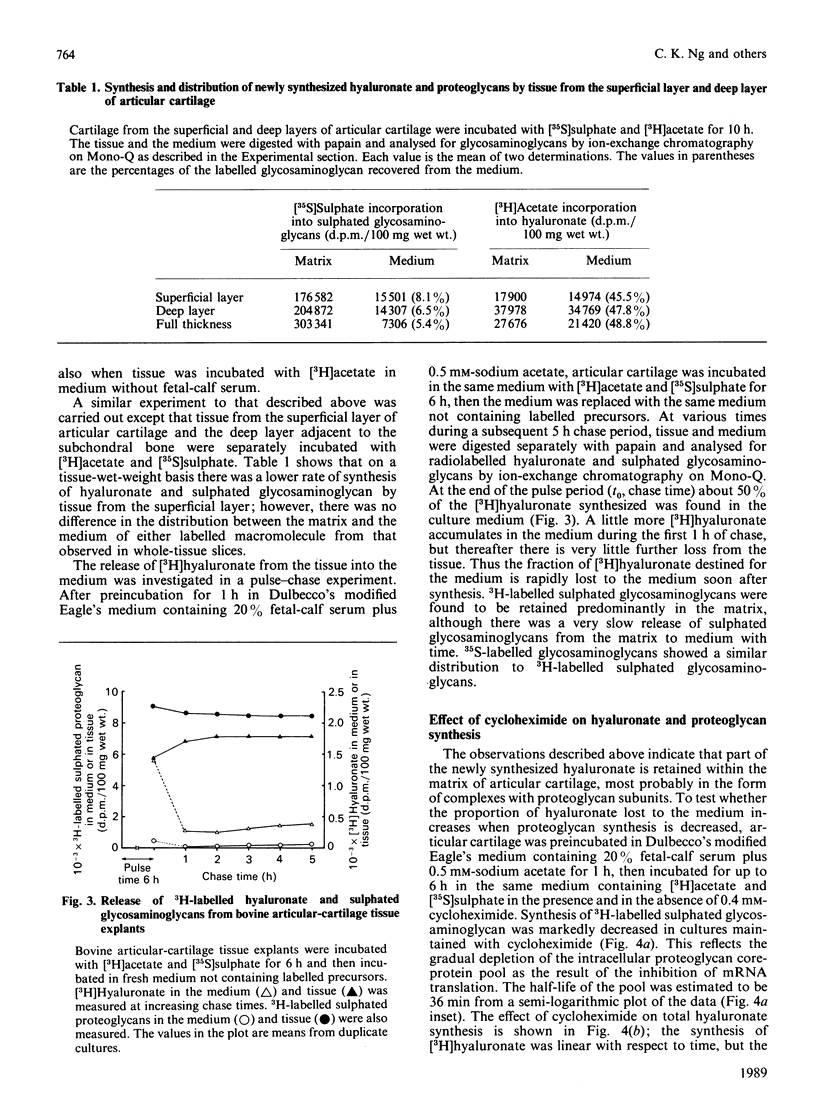
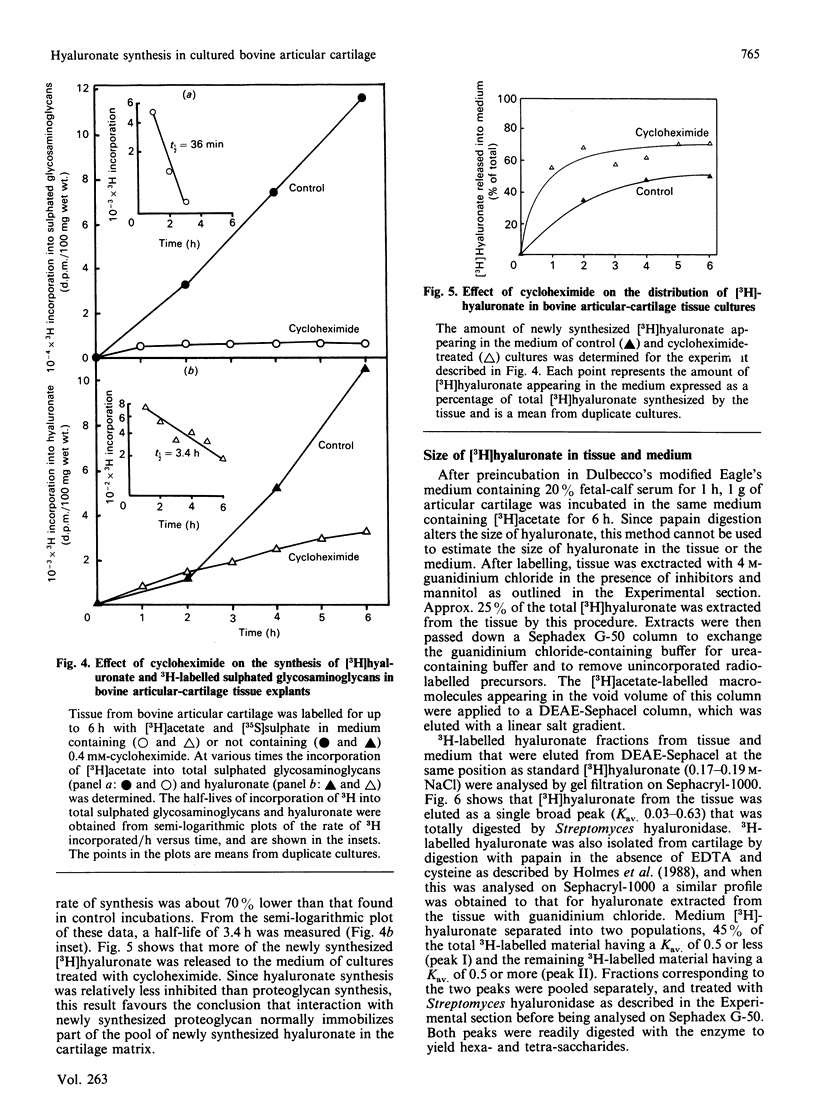
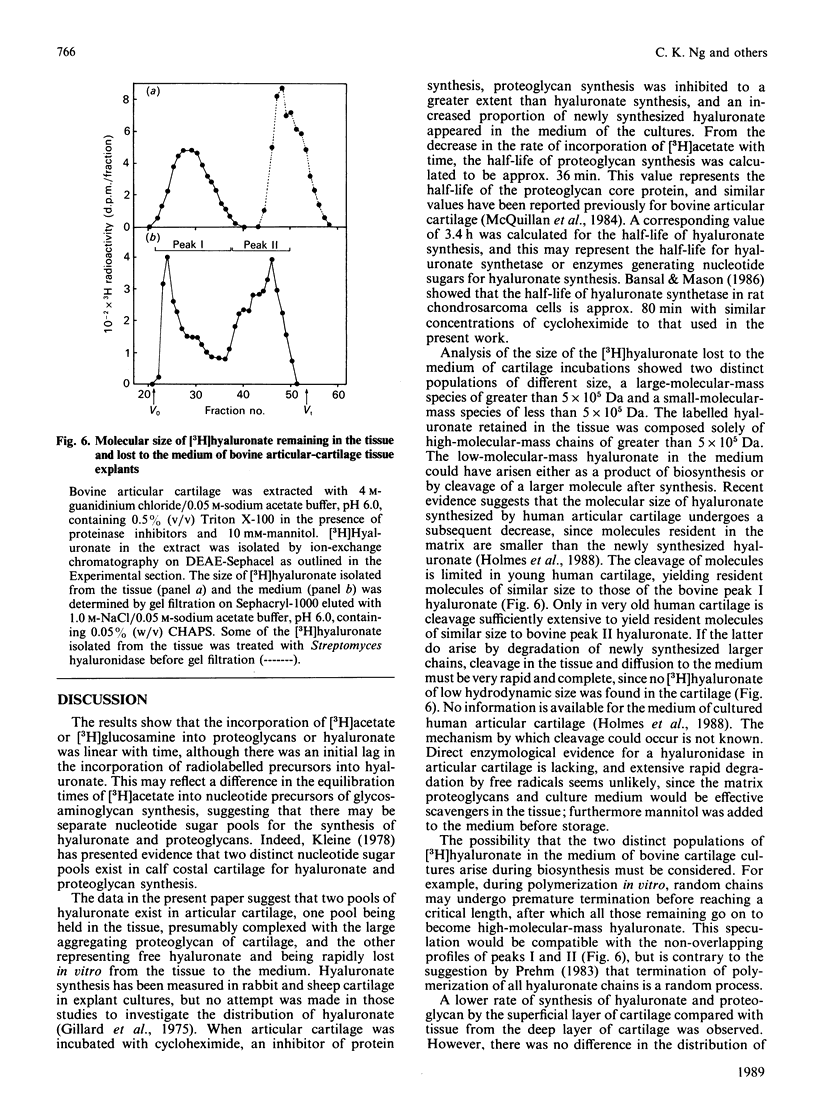
The synthesis and distribution of hyaluronate and proteoglycan were studied in bovine articular cartilage in short-term explant culture with [3H]acetate and H2(35)SO4 as precursors. The incorporation of [3H]acetate into hyaluronate and sulphated glycosaminoglycans was linear with time, except that hyaluronate synthesis showed a marked lag at the beginning of the incubation. [3H]Hyaluronate represented 4-7% of the total [3H]glycosaminoglycans synthesized over a 6 h period. However, the distributions of [3H]hyaluronate and 3H-labelled sulphated glycosaminoglycans were different: about 50% of the newly synthesized [3H]hyaluronate appeared in the medium, compared with less than 5% of the 3H-labelled sulphated proteoglycans. A pulse-chase experiment revealed that the release of newly synthesized [3H]hyaluronate from cartilage was rapid. No difference was observed in the distribution of [3H]hyaluronate between medium and tissue by cartilage from either the superficial layer or the deep layer of articular cartilage. When articular cartilage was incubated with 0.4 mM-cycloheximide, proteoglycan synthesis was markedly inhibited, whereas the synthesis of hyaluronate was only partially inhibited and resulted in more of the newly synthesized hyaluronate being released into the medium. Analysis of the hydrodynamic size of [3H]hyaluronate isolated from cartilage on Sephacryl-1000 revealed one population that was eluted as a broad peak (Kav. less than 0.7), compared with two populations (Kav. greater than 0.5 and less than 0.5) appearing in the medium of cultures. These data suggest that hyaluronate is synthesized in excess of proteoglycan synthesis and that the hyaluronate that is not complexed with proteoglycans is rapidly lost from the tissue.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bansal M. K., Mason R. M. Evidence for rapid metabolic turnover of hyaluronate synthetase in Swarm rat chondrosarcoma chondrocytes. Biochem J. 1986 Jun 1;236(2):515–519. doi: 10.1042/bj2360515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates E. J., Lowther D. A., Handley C. J. Oxygen free-radicals mediate an inhibition of proteoglycan synthesis in cultured articular cartilage. Ann Rheum Dis. 1984 Jun;43(3):462–469. doi: 10.1136/ard.43.3.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillard G. C., Caterson B., Lowther D. A. The synthesis of hyaluronic acid by sheep and rabbit articular cartilage in vitro. Biochem J. 1975 Feb;145(2):209–213. doi: 10.1042/bj1450209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handley C. J., Lowther D. A. Extracellular matrix metabolism by chondrocytes. III. Modulation of proteoglycan synthesis by extracellular levels of proteoglycan in cartilage cells in culture. Biochim Biophys Acta. 1977 Nov 7;500(1):132–139. doi: 10.1016/0304-4165(77)90053-8. [DOI] [PubMed] [Google Scholar]
- Handley C. J., Lowther D. A., McQuillan D. J. The structure and synthesis of proteoglycans of articular cartilage. Cell Biol Int Rep. 1985 Sep;9(9):753–782. doi: 10.1016/0309-1651(85)90095-5. [DOI] [PubMed] [Google Scholar]
- Hascall V. C., Handley C. J., McQuillan D. J., Hascall G. K., Robinson H. C., Lowther D. A. The effect of serum on biosynthesis of proteoglycans by bovine articular cartilage in culture. Arch Biochem Biophys. 1983 Jul 1;224(1):206–223. doi: 10.1016/0003-9861(83)90205-9. [DOI] [PubMed] [Google Scholar]
- Hascall V. C., Kimura J. H. Proteoglycans: isolation and characterization. Methods Enzymol. 1982;82(Pt A):769–800. doi: 10.1016/0076-6879(82)82102-2. [DOI] [PubMed] [Google Scholar]
- Holmes M. W., Bayliss M. T., Muir H. Hyaluronic acid in human articular cartilage. Age-related changes in content and size. Biochem J. 1988 Mar 1;250(2):435–441. doi: 10.1042/bj2500435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleine T. O. Hyaluronate-proteoglycan complex: evidence for separate biosynthesis mechanisms of the macromolecules. Connect Tissue Res. 1978;5(4):195–199. doi: 10.3109/03008207809152272. [DOI] [PubMed] [Google Scholar]
- Mason R. M., Kimura J. H., Hascall V. C. Biosynthesis of hyaluronic acid in cultures of chondrocytes from the Swarm rat chondrosarcoma. J Biol Chem. 1982 Mar 10;257(5):2236–2245. [PubMed] [Google Scholar]
- McQuillan D. J., Handley C. J., Robinson H. C., Ng K., Tzaicos C. The relation of RNA synthesis to chondroitin sulphate biosynthesis in cultured bovine cartilage. Biochem J. 1986 Apr 15;235(2):499–505. doi: 10.1042/bj2350499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prehm P. Hyaluronate is synthesized at plasma membranes. Biochem J. 1984 Jun 1;220(2):597–600. doi: 10.1042/bj2200597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prehm P. Synthesis of hyaluronate in differentiated teratocarcinoma cells. Characterization of the synthase. Biochem J. 1983 Apr 1;211(1):181–189. doi: 10.1042/bj2110181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagishita M., Hascall V. C. Effects of monensin on the synthesis, transport, and intracellular degradation of proteoglycans in rat ovarian granulosa cells in culture. J Biol Chem. 1985 May 10;260(9):5445–5455. [PubMed] [Google Scholar]


