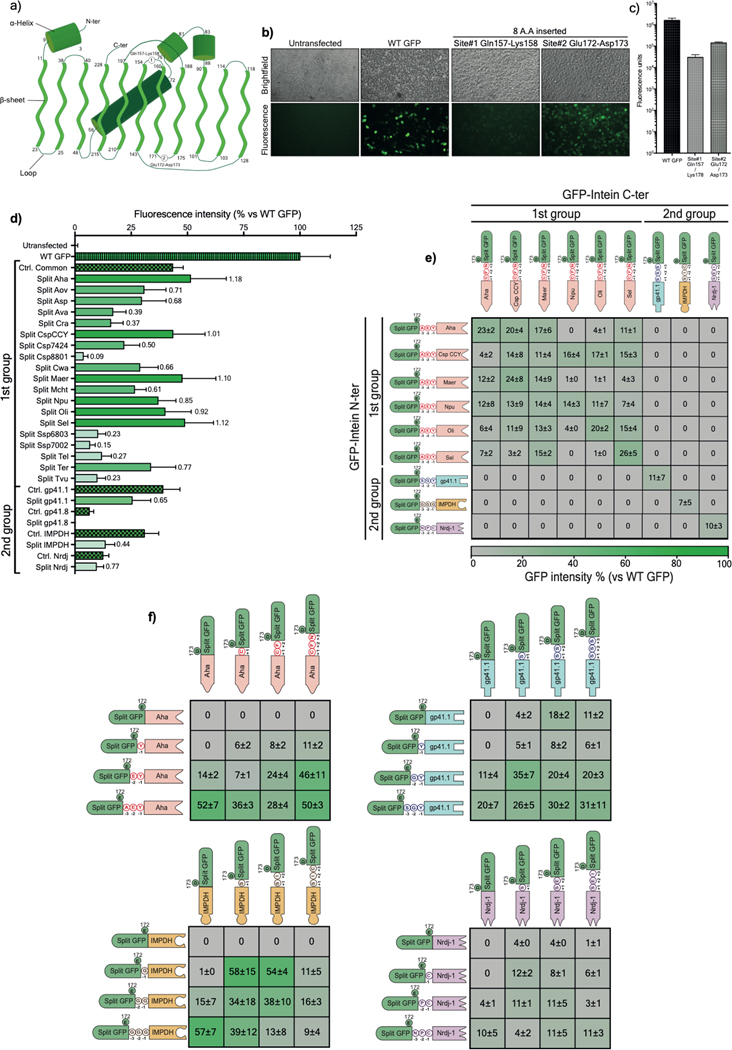
Extended Data Figure 1: Validation of GFP as a platform for split intein screening.
a) Diagram illustrating the topology of the GFP folding pattern with the chromophore, alpha helices, beta sheets, and the connecting loops with the two tested splitting sites (site#1: Glutamine 157-Lysine158, site#2: Glutamic acid 172-Aspartic acid 173). The numbers represent the delimiting residues at the beginning and the end of the secondary structures. b) Brightfield and fluorescent microscopy pictures of living HEK293 cells transfected with the WT or mutated GFP. c) Mean fluorescence intensities of transfected HEK293 cells with either WT GFP or two mutated GFP harboring 8-amino acid insertions within the tested sites. Site#2 was more permissive to the insertion of an octapeptide and, thus, was selected as a splitting site where different split inteins were inserted for initial screening. d) GFP fluorescence intensity measured on living HEK293 cells 24h post-transfection with a single plasmid expressing either WT GFP, control (ctrl) GFP with a six-residue footprint (checkered bars), or a dual plasmid expressing split intein/GFP. The protein ligation efficiency of each split intein (ratio of GFP fluorescence of a given intein to its internal control) is labeled on each bar (n=6). e) Characterization of the orthogonality of the preselected split inteins. The matrix shows cross-reactivity between split inteins from Group 1 but high specificity with pairs from Group 2. f) Determination of minimal extein AA required for efficient PTS of the top four split inteins using a combination of intein halves with variable linkers (n=3). Values are represented as an average of independent transfections ± s.e.m, that are normalized to values from cells transfected with WT GFP.