Abstract
Background.
Pancreatogenic diabetes, a consequence of pancreatic tissue loss following pancreatectomy, poses a significant challenge for patients undergoing pancreatic surgery. Islet autotransplantation (IAT) offers a promising approach to prevent or alleviate pancreatogenic diabetes, but its application has been limited to individuals with painful chronic pancreatitis.
Methods.
This study presents a 15-y clinical experience with the Milan Protocol, which expands IAT after pancreatectomy to a broader spectrum of patients with malignant and nonmalignant pancreatic diseases. The analysis evaluates feasibility, efficacy, and safety of IAT. Modified Igls criteria validated through the arginine test and mixed meal tolerance tests were used to assess long-term metabolic outcomes.
Results.
Between November 2008 and June 2023, IAT procedures were performed on 114 of 147 candidates. IAT-related complications occurred in 19 of 114 patients (16.7%), with 5 being potentially serious. Patients exhibited sustained C-peptide secretion over the 10-y follow-up period, demonstrating a prevalence of optimal and good beta-cell function. Individuals who underwent partial pancreatectomy demonstrated superior metabolic outcomes, including sustained C-peptide secretion and a reduced risk of developing diabetes or insulin dependence compared with those who underwent total pancreatectomy. For patients who had total pancreatectomy, the quantity of infused islets and tissue volume were identified as critical factors influencing metabolic outcomes. An increased risk of recurrence or progression of baseline diseases was not observed in subjects with neoplasms.
Conclusions.
These findings provide valuable insights into the benefits and applications of IAT as a therapeutic option for pancreatogenic diabetes after pancreatic surgery, expanding its potential beyond painful chronic pancreatitis.
INTRODUCTION
Pancreatogenic diabetes, a severe disruption of glucose homeostasis resulting from the loss of pancreatic parenchyma following pancreatectomy,1 has gained increased attention because of the rising number of pancreatectomies performed for benign, low-grade malignant, and malignant tumors.2
The development of pancreatogenic diabetes following partial or total pancreatectomy can have significant implications for patients, impacting both short-term and long-term outcomes in terms of glycemic control, quality of life, and overall survival.3-5 In response to this challenge, islet autotransplantation (IAT) has emerged as a promising therapeutic option, potentially preserving endocrine pancreatic function and mitigating the risk of postsurgery diabetes development.6-9 Initially used for patients with chronic pancreatitis,10,11 IAT has demonstrated efficacy in preserving beta-cell function and improving glycemic control.12-15 The Milan Protocol represents a significant advancement in the field of IAT, aiming to extend its application to a wider patient population with both malignant and nonmalignant pancreatic diseases,16-20 including those undergoing completion pancreatectomy because of anastomosis leakage after pancreaticoduodenectomy, those with high-risk pancreatic anastomosis during pancreaticoduodenectomy, and those undergoing extended left pancreatectomy for neoplasms located at the pancreatic isthmus.15,21-23 In this article, we present the experience of the Milan Protocol, providing a comprehensive analysis of a 15-y clinical program. We analyzed the long-term metabolic follow-up, as it plays a crucial role in assessing the durability of effects of IAT on glycemic control and the preservation of endocrine pancreatic function. Additionally, we address concerns regarding the potential dissemination of occult carcinoma cells. By shedding light on these critical aspects, we aim to contribute to the ongoing discussions surrounding the extension of IAT indications and its role in the management of pancreatogenic diabetes.
MATERIALS AND METHODS
Study Population and Eligibility Criteria
From November 2008 to June 2023, the Pancreatic Unit at S. Raffaele Scientific Institute in Milan, Italy, assessed all patients undergoing pancreas surgery for potential IAT. The Islet Processing Facility also served as a central hub for islet isolation upon request from collaborating institutions in the surrounding regions, including Milan (20 km away), Padova (247 km away), Pisa (280 km away), and Brescia (150 km away). Eligibility for IAT was determined on the basis of previously established criteria.16-20 In summary, eligible participants were adults with fasting blood glucose levels <126 mg/dL who had one of the following medical conditions: painful chronic pancreatitis, severe complications following pancreatic surgery, high-risk pancreaticoduodenectomy, and extensive distal pancreatectomy for benign/borderline neoplasms. Patients were excluded from the study if they had multiple pancreatic neoplasms, pathological involvement of the pancreatic transection margin, or any medical conditions that could hinder the safe completion of IAT. Before surgery, all participants underwent comprehensive preoperative assessments, typically including abdominal ultrasonography, contrast-enhanced computed tomography scans, endoscopic ultrasound, and additional imaging studies as deemed necessary. Informed consent was obtained from all participants, and the study protocol received ethical committee approval for the assessment and follow-up of transplantation procedures (NCT01702051).
Pancreas Collection and Islet Isolation
Pancreas procurement was performed using either open or laparoscopic surgical techniques under general anesthesia. The extent of resection varied from extensive left pancreatectomy to total pancreatectomy or completion pancreatectomy. The blood supply to the pancreas was preserved to minimize warm ischemia time for the islets. The decision to retain or remove the spleen was based on individual patient factors. In cases where pancreatic resection was performed because of a tumor, a 1 cm margin of the pancreatic remnant adjacent to the incision was excised and sent for frozen section examination to ensure complete tumor removal. The remaining segments of the pancreas were transported to the Islet Processing Facility in the cold University of Wisconsin preservation solution. The islet isolation and purification process followed the automated method initially introduced by Ricordi for allotransplantation, with local adaptations as previously described.16,17,21,24 In summary, the pancreatic duct was cannulated, and the pancreas was enzymatically digested using collagenase NB1 and neutral protease. The digested tissue underwent purification using a continuous gradient of Hanks’ balanced salt solution-Ficoll on a cell separator. The resulting purified islet fractions were then combined and pooled in Connaught Medical Research Laboratories 1066 medium. The purified islets were then reinfused via the portal vein, either intraoperatively if the clinical and logistical conditions allowed or within 48 h through percutaneous transhepatic cannulation of the portal vein. In cases where portal vein infusion was not possible, islets were infused into the bone marrow at the superoposterior iliac crest, as previously described.24
Follow-up
Follow-up appointments were scheduled for outpatients at 1, 3, 6, and 12 mo, and then annually after the index surgery. For patients diagnosed with malignancy, adjuvant chemotherapy or radiotherapy was administered as deemed necessary, and computed tomography scans and neoplastic marker tests were conducted every 3 or 6 mo, tailored to the individual’s risk of recurrence. Adverse events arising from the islet infusion procedure were documented and classified on the basis of the “Terminology Criteria for Adverse Events In Trials of Adult Pancreatic Islet Transplantation Version 4.1” (http://www.isletstudy.org/CITDocs/CIT-TCAE%20V4.pdf). Disease-specific, disease-free, diabetes-free, and insulin-free survival rates were recorded. Clinical biochemistry parameters were evaluated using previously described methods.25 The updated homeostasis assessment model of insulin resistance and secretion was calculated using freely available online software (https://www.dtu.ox.ac.uk/homacalculator/). The estimated glomerular filtration rate was calculated using the Modification of Diet in Renal Disease study equation. For each IAT case at each time point in our population, we assessed the outcomes for modified Igls criteria (Table 1).26 Additional metabolic tests were conducted during the follow-up period, with the consent of the recipients. The arginine test (involving the administration of 30 g of arginine hydrochloride for 30 min) was performed under fasting conditions, following overnight withdrawal of insulin therapy. Blood samples were collected to measure insulin, glucose, and C-peptide concentrations at baseline, and at 5, 10, 20, 30, 40, 50, 60, 90, and 120 min. The Milan group has widely used this protocol to evaluate both first-phase and second-phase insulin responses in pancreas or beta-cell transplant recipients.25,27 The first-phase insulin response (acute insulin response to arginine) was calculated as the incremental area under the insulin curve between 0 and 10 min. The overall pancreatic beta-cell response was assessed by calculating the area under the curve (AUC) of C-peptide during the 120-min test. Mixed meal tolerance tests (MMTTs) were performed using a test meal of 250 kcal (approximately 52% carbohydrates, 11% fats, and 37% proteins; Boost High Protein Rich Chocolate Balanced Nutritional Drink, Nestlé Health Science). The Boost drink was consumed within 10 min, and blood samples were collected at specific time intervals (–10, 0, 10, 20, 30, 60, 90, 120, and 180 min) after meal. Similar to the arginine test, the overall pancreatic beta-cell response to the mixed meal challenge was evaluated by calculating the AUC of C-peptide during the 120-min test. The highest C-peptide measurement recorded during the test was also identified as peak C-peptide.
TABLE 1.
Modified Igls criteria for metabolic classification of IAT
| HbA1c | SHE (per year)a | Insulin dose | Fasting C-peptideb | |
|---|---|---|---|---|
| Optimal | <6.5% | None | 0 U/kg/d | >0.5 ng/mL |
| Good | <7% | None | <0.5 U/kg/d | >0.5 ng/mL |
| Marginal | ≥7% | ≥1 | ≥0.5 U/kg/d | >0.3 ng/mL |
| Failed | – | – | – | ≤0.3 ng/m |
Any occurrence in the past year of hypoglycemia resulting in loss of consciousness or seizure.
For the assessment of fasting C-peptide, we adapted the original Igls criterion by considering the threshold for stimulated C-peptide.
HbA1c, glycated hemoglobin; IAT, islet autotransplantation; SHE, severe hypoglycemia event.
Statistical Analysis
Depending on appropriateness, categorical variables were compared using either the chi-squared test or Fisher exact test. For variables with a normal distribution (expressed as mean ± SD), we used the unpaired Student t test and ANOVA with post hoc Bonferroni test for comparisons. In contrast, variables with a nonnormal distribution (expressed as median and interquartile range) were analyzed using the Mann-Whitney U test or Kruskal-Wallis test with post hoc Dunn’s multiple comparison test. Survival analysis was conducted using the Kaplan-Meier method. Adjusted hazard ratios (HRs) and odds ratios (ORs), accounting for age and sex, were calculated using Cox proportional hazard and logistic regression, respectively. Multivariate analysis included variables that were significant in the univariate analysis, ensuring the exclusion of redundancy and overfitting risk. Two-tailed P values were reported, with a significance level of <0.05. Confidence intervals (CIs) were 2-sided and not adjusted for multiple testing unless otherwise specified. Statistical analyses were performed using SPSS version 24 (SPSS Inc/IBM) and GraphPad Prism version 5.04.
RESULTS
Patients, Surgery, and Islet Transplantation
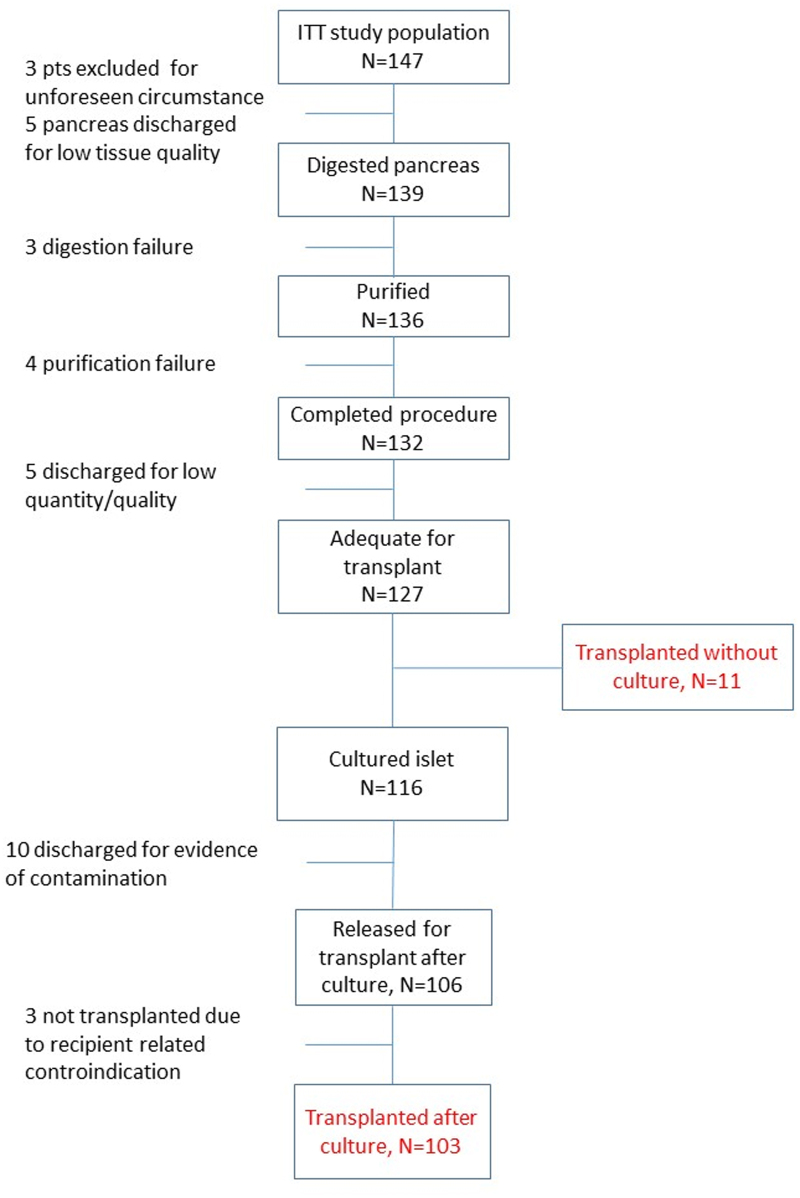
Between November 2008 and June 2023, a total of 147 patients were considered as candidates for IAT. Patient characteristics, diagnosis, and indications for IAT are summarized in Table 2 and Figure S1 and Table S1 (SDC, http://links.lww.com/TP/D50). The IAT procedure was performed on 114 of 147 candidates, accounting for 77.6% of the total, and patient disposition is reported in Figure 1. In 8 patients (5.4%), the pancreata were not processed because of issues with tissue quality (n = 5) or unforeseen circumstances (n = 3), such as a retired consensus or unavailability of technical facilities. Among the remaining patients, 22 (15%) had islet preparations that were deemed inadequate for transplantation after isolation, either because of a low quantity/quality of islets (n = 12) or bacterial contamination (n = 10). Additionally, 3 patients (2%) were not transplanted because of contraindications at the time of infusion: one experienced clinical instability following a cardiac arrest during surgery after pancreas resection, another had a severe hemobilia resulting from prior percutaneous biliary drainage for a biliary fistula, and the third presented with ischemic damage in the second and third segment of the left hepatic lobe. Of the 114 patients who underwent the IAT procedure (see Table 3), 11 individuals received fresh islets (9.6%), whereas 103 patients received cultured islets (90.4%). The cultured islets had a median culture time of 15 h (range, 14–16 h). The mean body weight of the recipients was 71.7 ± 15.5 kg, resulting in a median transplantation value of 1539 (1031–2114) islet equivalent (IE)/kg. The volume of islet tissue infused during the procedure was 1.5 mL (range, 1–2.5 mL) with a purification rate of 40% (range, 20%–60%). In 107 recipients (93.9%), the portal vein was used as the transplantation site, whereas in 7 recipients (6.1%), the bone marrow was used. The change in portal vein pressure following the infusion of islets was clinically insignificant. The median value before infusion was 12 (10–15) cm H2O, and the median value after infusion was 13 (11–16) cm H2O, resulting in a delta value of 1 cm H2O (P < 0.001).
TABLE 2.
Patient characteristics according to IAT indication
| Characteristics | Simultaneous IAT after pancreatectomy for chronic pancreatitis/trauma | Simultaneous IAT after total pancreatectomy | Simultaneous IAT after extended distal pancreatectomy | Salvage IAT after relaparotomy |
|---|---|---|---|---|
| Recruited, N | 14 | 65 | 41 | 27 |
| Center local/remote | 11/3 | 34/31 | 41/0 | 21/6 |
| Age, y | 45.6 ± 16 | 68.3 ± 10.1 | 53 ± 15.2 | 58.4 ± 16.8 |
| Sex (M/F) | 9/5 | 49/16 | 15/26 | 18/9 |
| Weight, kg | 66.5 ± 8.3 | 74.8 ± 13.9 | 72.3 ± 17.7 | 71.3 ± 17.8 |
| BMI, kg/m2 | 22.3 ± 3.3 | 26.4 ± 4.3 | 25.3 ± 4.7 | 25.8 ± 4 |
| EGFR, mL/min/1.73 m2 | 106.3 ± 25 | 85.9 ± 36.8 | 99.7 ± 27 | 80.9 ± 37 |
| Glucose, mg/dL | 101 ± 18 | 98 ± 16 | 86 ± 18 | 106 ± 36 |
| HbA1c, % | 5.5 ± 0.6 | 5.38 ± 0.64 | 5.28 ± 0.45 | 5.66 ± 0.49 |
| Insulin, mU/mL | 5.15 (4.3–18) | 9.1 (4.6–15.8) | 9.05 (5.6–16.2) | 6.9 (4.3–9.7) |
| C-peptide, ng/mL | 1.38 (0.77–3.7) | 2.5 (1.5–3.9) | 2.21 (1.48–3.23) | 2 (1.75–2.53) |
| Insulin HOMA2-IR | 0.78 (0.66–2.78) | 1.45 (0.82–2.71) | 1.33 (0.78–2.43) | 1.05 (0.75–1.36) |
| C-peptide HOMA2 %B | 81.7 (54.99 | 140 (98–164) | 139.7 (102–186) | 137.2 (111–172) |
| White blood cell, ×109/L | 8.9 (6.6–16.12) | 7.5 (6.5–12.4) | 10.3 (6.45–13.3) | 17 (13.7–24.2) |
| Hemoglobin, g/dL | 12.7 (11.1–13) | 11.7 (10.8–13.4) | 12.3 (11.3–13.1) | 10.1 (8.9–10.6) |
| Planned surgery | ||||
| Pancreaticoduodenectomy | 2 | 24 | 0 | 25 |
| (Sub)total pancreatectomy | 10 | 41 | 0 | 0 |
| Distal pancreatectomy | 2 | 0 | 37 | 0 |
| Middle pancreatectomy | 0 | 0 | 4 | 0 |
| Enucleation | 0 | 0 | 0 | 2 |
| Definitive surgery: total/subtotal/partial | 10/2/2 | 65/0/0 | 0/1/40 | 24/0/3 |
| Time from first surgery to relaparotomy, d | – | – | 13 (8–22) | |
| Splenectomy | 10 (71.4) | 15 (23.1) | 26 (63) | 17 (62.9) |
| Transplanted | N = 8 (57.1) | N = 55 (84.6) | N = 36 (87.8) | N = 15 (55.5) |
BMI, body mass index; EGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; HOMA, homeostasis model assessment; IAT, islet autotransplantation; IR, insulin resistance.
FIGURE 1.
Patient disposition for islet isolation during the study. ITT, intention to treat.
TABLE 3.
Patient characteristics according to pancreatectomy extension
| All (N = 114) | Total (N = 74) | Partial (N = 40) | P | |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Age, y | 61.5 (49–71) | 67 (55.8–73.2) | 51 (42.3–62) | <0.001 |
| Sex (M/F) | 70/44 | 54/20 | 16/24 | 0.001 |
| Weight, kg | 70 (63–80) | 72.5 (65–80) | 67 (59.2–77.2) | 0.12 |
| BMI, kg/m2 | 24.6 (22.8–28.2) | 25.4 (23.2–28.4) | 24.1 (21.7–27.5) | 0.1 |
| EGFR, mL/min/1.73 m2 | 89.5 (69.25–113) | 83 (60.5–115) | 96.5 (83–113) | 0.033 |
| Glucose, mg/dL | 92 (82–103) | 96 (84–104) | 88 (79–96) | 0.003 |
| HbA1c, % | 5.4 (5–5.8) | 5.4 (5–5.8) | 5.3 (5–5.7) | 0.53 |
| Insulin, mU/mL | 7.7 (5.5–14.8) | 7.5 (5.1–13.4) | 8.7 (5.6–16.3) | 0.408 |
| C-peptide, ng/mL | 2.17 (1.5–3.2) | 2.26 (1.5–3.24) | 2.02 (1.5–3.2) | 0.34 |
| Insulin HOMA2-IR | 1.2 (0.78–2.10) | 1.1 (0.79–1.97) | 1.27 (0.78–2.6) | 0.73 |
| C-peptide HOMA2 %B | 138 (101–166) | 145 (103–164) | 133 (100–179) | 0.75 |
| White blood cell, ×109/L | 9900 (7200–13 800) |
8300 (6900–14 800) |
10 850 (8125–13 750) |
0.31 |
| Hemoglobin, g/dL | 11.8 (10.4–13.1) | 11.3 (10.1–13.2) | 12.3 (11.3–13) | 0.2 |
| Baseline disease | ||||
| Malignant neoplasia | 75 (65.8) | 58 (78.4) | 17 (42.5) | <0.001 |
| Periampullary adenocarcinoma | 50 (43.9) | 50 (67.6) | 0 (0) | <0.001 |
| AdenoK ductal | 16 (14) | 16 (21.6) | 0 (0) | 0.001 |
| Endocrine tumor | 23 (20.2) | 6 (8.1) | 17 (42.5) | <0.001 |
| Pancreatitis | 8 (7) | 7 (9.5) | 1 (2.5) | 0.26 |
| Other diagnosis | 33 (28.9) | 11 (14.9) | 22 (55) | <0.001 |
| Surgery-related procedure | ||||
| Splenectomy | 46 (40.4) | 22 (29.7) | 24 (60) | 0.003 |
| Relaparotomy | 15 (13.2) | 13 (17.6) | 2 (5) | 0.081 |
| Pre-IAT biliary endoprosthesis | 34 (29.8) | 33 (44.6) | 1 (2.5) | <0.001 |
| Post-IAT adjuvant therapies | 38 (30.7) | 35 (47.3) | 3 (7.5) | <0.001 |
| Islet preparation | ||||
| Pancreas weight, g | 59 (38.7–77.1) | 70 (55–82) | 37 (28.2–49.2) | <0.001 |
| Islet yield (IA × 103) | 203.87 (145.12–256.1) |
210 (163.75–282.5) |
181.25 (122.42–235.62) |
0.045 |
| Islet yield (IA)/kg body weight | 2850 (2104–3619) |
3038 (2272–3706) |
2475 (1571–3596) |
0.066 |
| Islet yield (IEQ × 103) | 106.46 (72.35–151.30) |
119.66 (88.5–162.56) |
89.57 (47.71–106.44) |
<0.001 |
| Islet yield (IEQ)/kg body weight | 1546 (1060–2120) |
1694 (1243–2191) |
1113 (676–1606) |
0.001 |
| Isolation index | 0.54 (0.42–0.93) |
0.61 (0.43–0.77) |
0.47 (0.36–0.64) |
0.032 |
| In vitro culture | 103 (90.4) | 64 (86.5) | 39 (97.5) | 0.093 |
| Time of culture, h | 15 (13–16) | 14 (12–16) | 16 (15–17) | 0.001 |
| Purity | 40 (20–60) | 40 (20–60) | 35 (20–60) | 0.82 |
| Tissue volume, mL | 1.5 (1–2.5) | 1.5 (1–2.5) | 1.4 (0.55–2.73) | 0.25 |
| Islet infusion-related procedure | ||||
| Intraportal infusion | 107 (93.9) | 67 (90.5) | 40 (100) | 0.094 |
| Change in portal vein pressure | 1 (0–2) | 1 (0–2) | 1 (0–1) | 0.32 |
| Islet infusion complication | 19 (16.7) | 15 (20.3) | 4 (10) | 0.19 |
| Any portal vein thrombosis | 6 (5.3) | 5 (6.8) | 1 (2.5) | 0.66 |
| Any bleeding | 12 (10.5) | 9 (12.2) | 3 (7.5) | 0.53 |
| Microbial contamination | 18 (15.8) | 15 (20.3) | 3 (7.5) | 0.106 |
BMI, body mass index; EGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; HOMA, homeostasis model assessment; IA, islet absolute number; IAT, islet autotransplantation; IEQ, islet equivalent quantity; IR, insulin resistance.
Of the 114 patients who underwent islet infusion, 19 (16.7%) experienced complications related to the procedure. Among these complications, 5 were classified as potentially serious with a Terminology Criteria for Adverse Events score >3. Specifically, 6 patients (5.3%) developed portal vein thrombosis (PVT), with 1 case involving total PVT and 5 cases involving left branch PVT. All of these patients received anticoagulation therapy with low-molecular-weight heparin, which successfully resolved the thrombosis. Additionally, 12 patients experienced bleeding related to the percutaneous portal vein access procedures. Eight of these bleeding events were not severe enough to require intervention, 3 cases required transfusion alone, and 1 case required both transfusion and surgery. Finally, 1 patient developed a liver abscess that necessitated long-term antibiotic treatment. A logistic regression analysis was conducted to determine the factors that influence a patient’s likelihood of receiving IAT (Figure 2). The analysis revealed several variables that were associated with a decreased probability of undergoing IAT. These factors included higher baseline glycated hemoglobin (HbA1c) levels (OR 0.39; 95% CI, 0.17-0.93; P = 0.033), salvage IAT following relaparotomy (OR 0.26; 95% CI, 0.11-0.65; P = 0.004), a history of biliary stent placement (OR 0.37; 95% CI, 0.17-0.84; P = 0.017), and a diagnosis of pancreatitis as the underlying disease (OR 0.24; 95% CI, 0.08-0.69; P = 0.008; Figure 2). The multivariate analysis further confirmed that a history of biliary stent placement (OR 0.31; 95% CI, 0.11-0.88; P = 0.027) and a diagnosis of chronic pancreatitis (OR 0.19; 95% CI, 0.05-0.69; P = 0.012) were independent predictors for a reduced likelihood of undergoing IAT.
FIGURE 2.
Probability of undergoing IAT as determined by logistic regression analysis. Univariate and multivariate odds ratios for IAT are presented. Logistic regression was used to evaluate the associations between patient characteristics and IAT. All presurgery variables that were analyzed are included. The dots represent the odds ratio after log transformation, whereas the lines indicate the 95% confidence intervals. ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CRP, C-reactive protein; EGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; Hb, hemoglobin; HbA1c, glycated hemoglobin; HOMA, homeostasis model assessment; IAT, islet autotransplantation; PLT, platelet; WBC, white blood cell.
Of the 114 patients who underwent pancreatic resection, 78 (68.4%) were still alive at the time of the final follow-up assessment (Figure 3). Among the deaths attributed to pancreatic surgery, 9 (6.2%) patients experienced fatal complications while still hospitalized. Their median survival period was 16 d. Additionally, 1 patient (0.9%) died from postoperative complications related to underlying comorbidities after discharge. Among the deaths not linked to pancreatic surgery, 17 (19.7%) occurred postdischarge, primarily because of the recurrence of the initial pancreatic neoplastic disease.
FIGURE 3.
Follow-up: overall survival. The probability of survival in patients receiving IAT is presented using Kaplan-Meier analysis (upper panel). The associations between patient characteristics and overall survival were evaluated using Cox regression analysis (lower panel). All presurgery variables that were analyzed are included in the analysis. The dots in the figure represent the hazard ratio after log transformation, and the lines represent the corresponding 95% confidence intervals. ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CRP, C-reactive protein; EGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; Hb, hemoglobin; HbA1c, glycated hemoglobin; HOMA, homeostasis model assessment; IE, islet equivalent; PLT, platelet; WBC, white blood cell.
Additionally, 6 (2.3%) patients died from other causes, including metachronous cancer, cardiovascular disease, and COVID-19 infection. The cause of death was unknown in 3 (2.6%) patients. The univariate analysis using Cox proportional hazards regression (Figure 3) showed that older age, higher basal transaminase levels, malignant neoplasia, head of pancreas localization, and a larger extent of pancreatectomy were associated with an increased risk of death. The multivariate analysis further confirmed that head of pancreas localization was an independent risk factor for death.
A subanalysis was conducted to assess the oncologic follow-up of patients with malignant neoplasia who underwent IAT. Of the 75 patients with malignant neoplasia, 37 (49.3%) received adjuvant chemotherapy or radiotherapy, and 54 (72%) were disease-free at the last follow-up. The recurrence of neoplasia occurred in 21 patients (28%; median 322 [95% CI, 226-417] d after surgery) with a distribution of local recurrence in 19% (median 432 [286-577] d), metastatic recurrence in 52.4% (median 322 [155-488] d), and simultaneous recurrence in 28.6% (median 225 [73-377] d). Among the 17 patients experiencing the systemic recurrence, 13 had liver metastasis (median 254 [40-367] d), 4 exhibited lung involvement (median 352 [299-404] d), 4 presented peritoneal recurrence (median 178 [0-437] d), and 1 displayed bone involvement (738 d). This recurrence pattern did not show significant differences between patients receiving IAT and those who did not (Table S2, SDC, http://links.lww.com/TP/D50).
Validation of Modified Igls Criteria
Metabolic outcomes were evaluated using the revised Igls criteria (Table 1),26 which categorize beta-cell graft function into 4 groups: optimal, good, marginal, and failed, based on HbA1c, severe hypoglycemia events, insulin requirements, and C-peptide levels. If any required information was unavailable at a given time, the outcome was labeled as “not evaluable.” The study followed 114 patients for a median of 6.3 y (interquartile range, 4.8–7.8 y). Of these, 78 (68.4%) were still alive at the last follow-up. The number of patients available for analysis at different follow-up intervals was 96 at 1 y, 51 at 5 y, and 20 at 10 y. To assess the validity of the modified Igls criteria, we analyzed data from 937 time points collected during the follow-up period (Figure 4). Due to incomplete data, 107 time points (11.4%) were excluded from the analysis. Among the remaining 830 time points, optimal beta-cell graft function (HbA1c 5.7% [5.3–5.9], fasting C-peptide 1.76 ng/mL [1.3–2.3], and insulin requirement 0 U/kg/d) was observed in 435 instances (46.4%). Notably, the majority of these optimal outcomes (64.3%) occurred within the initial years following IAT, with a gradual decline over time (Figure 4). Good beta-cell graft function (HbA1c 6.5% [5.9–6.7], fasting C-peptide 1.48 ng/mL [0.9–2.14], and insulin requirement 0.16 U/kg/d [0.01–0.28]) was observed in 136 instances, accounting for 14.5% of the total. Similar to optimal outcomes, good function was primarily observed during the first year after IAT (n = 98; 72%), followed by a decrease over time. Marginal beta-cell graft function (HbA1c 7.4% [7–8.2], fasting C-peptide 0.72 ng/mL [0.49–.52], and insulin requirement 0.36 U/kg/d [0.13–0.52]) was observed in 157 instances (16.7%). Failed beta-cell graft function (HbA1c 7.1% [6.3–8], fasting C-peptide 0.17 ng/mL [0.07–0.22], and insulin requirement 0.43 U/kg/d [0.35–0.6]) was observed in 102 instances (10.8%), exhibiting an increasing trend over time, in contrast to optimal and good function.
FIGURE 4.
Metabolic outcome of IAT according to Igls criteria. The β-cell graft function of 114 IAT cases was evaluated and classified into 4 categories: “optimal,” “good,” “marginal,” or “failure” based on the revised Igls criteria (Table 1). Of 204 arginine tests performed, 58, 58, 57, and 31 results fell into the categories of optimal, good, marginal, and failed beta-cell function, respectively. Similarly, among 169 MMTTs conducted, 106, 22, 26, and 15 results corresponded to optimal, good, marginal, and failed beta-cell function, respectively. The upper panel presents the β-cell graft function outcomes according to the Igls criteria during a 10-y follow-up period. The lower panels display the distribution of metabolic parameters using box plots. The centerline of the box plot represents the median value, and the box encompasses the interquartile range of the data set. The whiskers extend to the 1st and 99th percentiles. Values beyond these bounds are considered outliers and are depicted as black dots. Statistical analysis was conducted using the Kruskal-Wallis test, followed by post hoc Dunn’s multiple comparison test. The results of the analysis are as follows: aall groups showed statistically significant differences, except for the comparison between the “marginal” and “failed” groups; ball groups exhibited statistically significant differences from each other; call groups demonstrated statistically significant differences, except for the comparison between the “optimal” and “good” groups; and dthe “failed” group exhibited statistically significant differences from all other groups. HbA1c, glycated hemoglobin; IAT, islet autotransplantation; MMTT, mixed meal tolerance test.
The arginine test and MMTTs provided valuable standardized parameters for assessing the effectiveness of IAT, including measurements of stimulated insulin and C-peptide secretion. Significant differences in acute insulin secretion response to arginine and peak C-peptide secretion during the test were observed among the 4 metabolic categories. The 2-h C-peptide release AUC exhibited significant differences among the comparison groups, except between the optimal and good outcome groups. Fasting glucose levels aligned with HbA1c and varied among the groups, whereas fasting proinsulin levels were significantly lower only in the failure group. These findings validate the modified Igls Classification as an effective means of evaluating the metabolic outcome of IAT.
Metabolic Outcome of IAT According to the Extent of Pancreas Resection
A subsequent analysis was undertaken with the primary aim of preemptively differentiating between the 2 modalities of pancreatectomy: total pancreatectomy (n = 74) and partial pancreatectomy (n = 40). Table 2 provides comprehensive information on patient and islet characteristics according to the extent of pancreas resection. Partial pancreatectomy patients demonstrated excellent disease-free survival and overall survival as expected because of the selected indication for benign/borderline lesions and neuroendocrine tumors in the body or neck of the pancreas (Figure 5). Metabolically, partial pancreatectomy patients received fewer islets, as expected because of reduced pancreatic tissue availability for isolation. However, they demonstrated exceptional metabolic outcomes because of the residual naive pancreas contributing to glucose control maintenance. All partial pancreatectomy patients sustained C-peptide secretion for 10 y, with a high prevalence of optimal and good beta-cell function. They also had an extremely low risk of developing diabetes (HR 0.1; 95% CI, 0.05-0.18) or insulin dependency (HR 0.07; 95% CI, 0.04-0.13) compared with total pancreatectomy patients. Despite receiving more islets, total pancreatectomy patients had less favorable metabolic outcomes, with a gradual increase in marginal and failed beta-cell function over time. These patients also had an extremely lower probability of maintaining sustained C-peptide secretion (HR 0.10; 95% CI, 0.04-0.23) and graft survival (HR 0.11; 95% CI, 0.045-0.27) compared with partial pancreatectomy patients. Cox regression analysis was used to identify the factors influencing the maintenance of sustained C-peptide secretion and islet transplant survival in total pancreatectomized subjects (Figure 6). In the univariate analysis, a lower islet yield, particularly when expressed as IE quantity (IEQ) per kg of body weight (HR 0.48; 95% CI, 0.29-0.78; P = 0.003), a larger tissue volume (HR 1.19; 95% CI, 1.01-1.4; P = 0.039), and extrahepatic infusion site (HR 1.19; 95% CI, 1.01-1.4; P = 0.039) were associated with the loss of sustained C-peptide secretion. Multivariate analysis confirmed that lower islet yield and extrahepatic infusion site were independent risk factors for this outcome. Lower islet yield was significantly and independently associated with graft survival (Figure 6). Based on these findings, patients were divided into 3 groups based on the tertiles of their received islet yield: >2060 IEQ/kg, between 2060 and 1426 IEQ/kg, and <1426 IEQ/kg (Figure 7). Transplanting >2060 IEQ/kg resulted in a high likelihood of graft survival and sustained C-peptide secretion, leading to predominantly optimal and marginal metabolic outcomes. Conversely, transplanting <1426 IEQ/kg was associated with transient graft survival and function that generally declined over time. Patients who received between 2060 and 1426 IEQ/kg had intermediate outcomes.
FIGURE 5.
Metabolic outcome of IAT according to the extent of pancreas resection. The functional outcomes of β-cell replacement therapy were evaluated separately for the 2 distinct groups of patients who underwent different types of pancreatectomy: total pancreatectomy (n = 74) and partial pancreatectomy (n = 40). The upper panel displays the outcomes based on the Igls criteria during a 10-y follow-up period. The lower panels present Kaplan-Meier analyses, showcasing the overall, disease-free, diabetes-free, and insulin-free survival rates, along with the probability of sustained (>0.5 ng/mL) or minimal (>0.3 ng/mL) C-peptide secretion. IAT, islet autotransplantation.
FIGURE 6.
Metabolic follow-up: C-peptide secretion maintenance in totally pancreatectomized patients. Cox regression analysis was used to examine the associations between patient characteristics and the risk of losing C-peptide secretion. All presurgery variables that were analyzed are included. The dots in the figure represent the hazard ratio after log transformation, and the lines represent the corresponding 95% confidence intervals. ALT, alanine aminotransferase; AST, aspartate aminotransferase; BM, bone marrow; BMI, body mass index; CRP, C-reactive protein; EGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; Hb, hemoglobin; HbA1c, glycated hemoglobin; HOMA, homeostasis model assessment; IA, islet absolute number; IAT, islet autotransplantation; IEQ, islet equivalent quantity; PLT, platelet; WBC, white blood cell.
FIGURE 7.
Metabolic follow-up in totally pancreatectomized patients according to the received islet yield. Patients were categorized into 3 groups according to the received islet yield in tertiles: >2060 IEQ/kg (n = 25), between 2060 and 1426 IEQ/kg (n = 25), and <1426 IEQ/kg (n = 24). The left panel displays outcomes using Igls criteria during a 10-y follow-up. The right panels present Kaplan-Meier analysis for the probability of sustained (>0.5 ng/mL) or minimal (>0.3 ng/mL) C-peptide secretion. P values are from the log-rank (Mantel-Cox) test, assessing differences in C-peptide secretion among the groups. IEQ, islet equivalent quantity.
DISCUSSION
This article presents findings that contribute evidence to ongoing discussions9,22,28-30 regarding the expansion of IAT indications and its role in managing pancreatogenic diabetes and proposes multiple points of interest. First, the analysis demonstrates that IAT shows promising results in preserving beta-cell function and glycemic control, aligning with previous studies that have demonstrated the effectiveness of IAT in preserving beta-cell function and improving glucose homeostasis in patients with chronic pancreatitis.7,8,14,31 Over the 10-y follow-up period, patients displayed a sustained C-peptide secretion, and there was a significant prevalence of optimal and good beta-cell function. Moreover, the study underscores the importance of adequate islet yield and the significance of the extent of pancreatectomy in achieving better metabolic outcomes. Second, the study proposes modified Igls criteria26 to better assess beta-cell graft function in the context of IAT and validated them using arginine test and MMTT. Instead of using stimulated C-peptide values, we focused on fasting C-peptide values because this approach proves to be more accessible and circumvents the necessity for tests that patients frequently refuse or are unable to complete adequately, particularly in cases involving insulin treatment, underlying neoplastic disease, marginal transplant function, and varying degrees of exocrine secretion deficiency. Furthermore, we set the fasting C-peptide threshold at 0.5 ng/mL, distinguishing between good and marginal function, and at 0.3 ng/mL, distinguishing between marginal function and failure, instead of the original Igls thresholds of 0.2 ng/mL and 0.1 ng/mL, respectively. It is important to note that some critics might consider our thresholds too stringent because fasting C-peptide values ranging from 0.09 to 0.2 ng/mL have been reported as sufficient for protection against hypoglycemia32-34 and microvascular disease progression.35 When evaluating IAT function in patients undergoing pancreatic surgery according to the Milan Protocol, it is essential to consider the overall goal. The primary objective of pancreatic surgery is to treat the underlying pancreatic disease, and as such, patients with IAT can achieve a “successful” surgical outcome regardless of islet function. However, improved diabetes outcomes could potentially lead to better overall results, which would be desirable. Nonetheless, it is worth noting that the procedure is expensive, requires a high level of coordination, and is sometimes performed in emergency situations. Therefore, being more stringent in justifying the procedure is appropriate. Currently, 3 other studies have used modified Igls criteria to assess IAT outcomes.36-38 One notable study, conducted by the University of Minnesota,36 examined a large cohort of 379 patients who received 4264 IEQ/kg and underwent total pancreatectomy with IAT for chronic pancreatitis. At 1-y follow-up, 115 patients were excluded because of missing data, and of the patients who completed the 1-y follow-up, 36% had optimal outcomes, 37% had good outcomes, 24% had marginal outcomes, and 3% had failed outcomes. These results are consistent with our own, where patients who received the highest amount of islet infusion (2646 IEQ/kg) following total pancreatectomy and IAT achieved 1-y outcome of 38.8% optimal, 22.2% good, 33.3% marginal, and 5% failed.
Third, findings of this study underscore the viability and safety of IAT for a wider range of pancreatic surgery patients, encompassing those beyond chronic pancreatitis. The IAT procedure was successfully performed on 77.6% of eligible patients, and a centralized isolation facility seamlessly supplied islets to both local and remote surgical units throughout the study. This demonstrates the feasibility of multicenter IAT programs, even with expanded indications, as previously proposed for IAT in chronic pancreatitis.39,40 Additionally, the analysis identified several factors associated with the likelihood of undergoing IAT, providing valuable insights for enhanced patient selection in the future. The study identified procedure-related complications in 16.7% of patients, with potentially severe complications occurring at a rate of 4.3%. This rate is consistent with previous experiences with IAT and higher than expected on the basis of previous allotransplantation experience, likely because of the severity of concomitant illness and complex pancreatic surgical procedures. Moreover, results of this study confirm that IAT does not pose an increased risk of disease recurrence or metastasis in patients with malignant neoplasms, which appears in line with that expected for these patients on the basis of literature and our experience.41-43
The study has limitations worth acknowledging. Despite being the largest study of IAT for extended indications, the sample size is relatively small and the patient population is quite heterogeneous. This heterogeneity may limit the applicability of the findings to broader populations. Moreover, an available control group for comparative outcome analysis is lacking. One potential option could involve assessing patients initially considered for IAT but who did not undergo the procedure. However, we excluded this group from consideration, except when examining the recurrence pattern. The exclusion was made because the selection of patients for the non-IAT group was not randomized and various factors such as patient characteristics, disease severity, or physician discretion may have influenced the decision not to proceed with IAT. These factors could introduce biases, potentially affecting the comparison between the 2 groups and influencing the interpretation of the results.
In summary, the findings presented in this article contribute to our understanding of the potential benefits and challenges of IAT in managing pancreatogenic diabetes following pancreatic surgery. The results support the effectiveness of IAT in preserving endocrine function and improving glycemic control. Insights gained from the Milan Protocol experience can inform future research collaborations and guide the optimization of IAT indications. Further studies are necessary to validate these findings, explore the long-term outcomes of IAT, and refine patient selection criteria for this innovative therapeutic approach.
ACKNOWLEDGMENTS
The authors thank Paola Macchieraldo, Antonio Mincione, Luca Casaura, Patrizia Pappini Oldrati, Andrea Marchesi, and Michele Mainardi for supporting the fundraising campaign “Un brutto t1po.”
Supplementary Material
Footnotes
G.B. and A.Z. contributed equally to this work.
L.P., G.B., and A.Z. contributed to conceptualization, study design, and data interpretation. R.M., F.A., G.C., P.M. P.M., N.P., M.F., and F.G. contributed to data collection. R.N., A.M., R.F., R.C., D.C., C.G., F.D.C., and D.P. contributed to the methods. L.P., P.B., and A.Z. had access to raw data. R.M. contributed to data curation. L.P. analyzed the data acquired funding and wrote the original draft of the report. G.B., A.Z., and M.S. reviewed and edited the report. G.B., A.Z., and L.P. are responsible for final submission of the article for publication and accessed and verified the underlying study data. All authors approved the final version before submission.
The authors declare no conflicts of interest.
This work was in part funded by Italian Ministry of Health (Ricerca finalizzata RF-2009-1483387) and SOStegno 70 Insieme ai ragazzi diabetici Associazione Onlus (Project: Beta is better).
The funders had no opportunity to review the article, and the authors are solely responsible for the final content and interpretation of the collected data.
Individual participant data will not be made available. Study protocol, statistical analysis plan, and analytical code will be available from the time of publication in response to any reasonable request addressed to the corresponding author.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantjournal.com).
REFERENCES
- 1.Infante M, Ricordi C. The unique pathophysiological features of diabetes mellitus secondary to total pancreatectomy: proposal for a new classification distinct from diabetes of the exocrine pancreas. Exp Rev Endocrinol Metab. 2023;18:19–32. [DOI] [PubMed] [Google Scholar]
- 2.Nortunen M, Meriläinen S, Ylimartimo A, et al. Evolution of pancreatic surgery over time and effects of centralization—a single-center retrospective cohort study. J Gastroint Oncol. 2023;14:366–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reddy S, Wolfgang CL, Cameron JL, et al. Total pancreatectomy for pancreatic adenocarcinoma: evaluation of morbidity and long-term survival. Ann Surg. 2009;250:282–287. [DOI] [PubMed] [Google Scholar]
- 4.Hartwig W, Gluth A, Hinz U, et al. Total pancreatectomy for primary pancreatic neoplasms: renaissance of an unpopular operation. Ann Surg. 2015;261:537–546. [DOI] [PubMed] [Google Scholar]
- 5.Scavini M, Dugnani E, Pasquale V, et al. Diabetes after pancreatic surgery: novel issues. Curr Diab Rep. 2015;15:16. [DOI] [PubMed] [Google Scholar]
- 6.Aleotti F, Nano R, Piemonti L, et al. Total pancreatectomy sequelae and quality of life: results of islet autotransplantation as a possible mitigation strategy. Updates Surg. 2021;73:1237–1246. [DOI] [PubMed] [Google Scholar]
- 7.Turner KM, Delman AM, Donovan EC, et al. Total pancreatectomy and islet cell autotransplantation: a 10-year update on outcomes and assessment of long-term durability. HPB. 2022;24:2013–2021. [DOI] [PubMed] [Google Scholar]
- 8.Chinnakotla S, Beilman GJ, Vock D, et al. Intraportal islet autotransplantation independently improves quality of life after total pancreatectomy in patients with chronic refractory pancreatitis. Ann Surg. 2022;276:441–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ludwig S, Distler M, Schubert U, et al. Quality of life and metabolic outcomes after total pancreatectomy and simultaneous islet autotransplantation. Commun Med. 2022;2:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bellin MD, Freeman ML, Gelrud A, et al. ; PancreasFest Recommendation Conference Participants. Total pancreatectomy and islet autotransplantation in chronic pancreatitis: recommendations from PancreasFest. Pancreatology. 2014;14:27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dudeja V, Beilman GJ, Vickers SM. Total pancreatectomy with islet autotransplantation in patients with malignancy: are we there yet? Ann Surg. 2013;258:219–220. [DOI] [PubMed] [Google Scholar]
- 12.Nanno Y, Wastvedt S, Freeman ML, et al. Metabolic measures before surgery and long-term diabetes outcomes in recipients of total pancreatectomy and islet autotransplantation. Am J Transplant. 2021;21:3411–3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bellin MD, Beilman GJ, Sutherland DE, et al. How durable is total pancreatectomy and intraportal islet cell transplantation for treatment of chronic pancreatitis? J Am Coll Surg. 2019;228:329–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khazaaleh S, Babar S, Alomari M, et al. Outcomes of total pancreatectomy with islet autotransplantation: a systematic review and meta-analysis. World J Transplant. 2023;13:10–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balzano G, Zerbi A, Aleotti F, et al. Total pancreatectomy with islet autotransplantation as an alternative to high-risk pancreatojejunostomy after pancreaticoduodenectomy: a prospective randomized trial. Ann Surg. 2023;277:894–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balzano G, Maffi P, Nano R, et al. Extending indications for islet autotransplantation in pancreatic surgery. Ann Surg. 2013;258:210–218. [DOI] [PubMed] [Google Scholar]
- 17.Balzano G, Maffi P, Nano R, et al. Autologous islet transplantation in patients requiring pancreatectomy: a broader spectrum of indications beyond chronic pancreatitis. Am J Transplant. 2016;16:1812–1826. [DOI] [PubMed] [Google Scholar]
- 18.Balzano G, Maffi P, Nano R, et al. Diabetes-free survival after extended distal pancreatectomy and islet auto transplantation for benign or borderline/malignant lesions of the pancreas. Am J Transplant. 2019;19:920–928. [DOI] [PubMed] [Google Scholar]
- 19.Balzano G, Carvello M, Piemonti L, et al. Combined laparoscopic spleen-preserving distal pancreatectomy and islet autotransplantation for benign pancreatic neoplasm. World J Gastroenterol. 2014;20:4030–4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balzano G, Nano R, Maffi P, et al. Salvage islet auto transplantation after relaparatomy. Transplantation. 2017;101:2492–2500. [DOI] [PubMed] [Google Scholar]
- 21.Balzano G, Piemonti L. Autologous islet transplantation in patients requiring pancreatectomy for neoplasm. Curr Diab Rep. 2014;14:1–10. [DOI] [PubMed] [Google Scholar]
- 22.Chaouch MA, Leon P, Cassese G, et al. Total pancreatectomy with intraportal islet autotransplantation for pancreatic malignancies: a literature overview. Expert Opin Biol Ther. 2022;22:491–497. [DOI] [PubMed] [Google Scholar]
- 23.Baldwin XL, Williams BM, Schrope B, et al. What is new with total pancreatectomy and autologous islet cell transplantation? Review of current progress in the field. J Clin Med. 2021;10:2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maffi P, Balzano G, Ponzoni M, et al. Autologous pancreatic islet transplantation in human bone marrow. Diabetes. 2013;62:3523–3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caumo A, Maffi P, Nano R, et al. Comparative evaluation of simple indices of graft function after islet transplantation. Transplantation. 2011;92:815–821. [DOI] [PubMed] [Google Scholar]
- 26.Landstra CP, Andres A, Chetboun M, et al. Examination of the Igls criteria for defining functional outcomes of β-cell replacement therapy: IPITA Symposium Report. J Clin Endocrinol Metab. 2021;106:3049–3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Piatti PM, Pontiroli AE, Caumo A, et al. Hyperinsulinemia decreases second-phase but not first-phase arginine-induced insulin release in humans. Diabetes. 1994;43:1157–1163. [DOI] [PubMed] [Google Scholar]
- 28.Jabłońska B, Mrowiec S. Total pancreatectomy with autologous islet cell transplantation—the current indications. J Clin Med. 2021;10:2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miles CB, Gardner TB. Expanding indications for pancreatic islet cell transplantation. Curr Opin Gastroenterol. 2020;36:452–455. [DOI] [PubMed] [Google Scholar]
- 30.Balzano G, Zerbi A, Scavini M, et al. Response to: comment on: pancreatectomy with islet-autotransplantation as alternative for pancreato-duodenectomy in patients with a high-risk for postoperative pancreatic fistula: the jury is still out. Ann Surg Open. 2023;4:e261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lad SU, Ali KF, Johnston PC, et al. Follow-up of patients after total pancreatectomy and islet cell autotransplantation at off-site islet isolation facility. J Clin Endocrinol Metab. 2023;108:1425–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gibb FW, McKnight JA, Clarke C, et al. Preserved C-peptide secretion is associated with fewer low-glucose events and lower glucose variability on flash glucose monitoring in adults with type 1 diabetes. Diabetologia. 2020;63:906–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marren S, Hammersley S, McDonald T, et al. Persistent C-peptide is associated with reduced hypoglycaemia but not HbA1c in adults with longstanding type 1 diabetes: evidence for lack of intensive treatment in UK clinical practice? Diabet Med. 2019;36:1092–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sorensen J, Johannesen J, Pociot F, et al. ; Danish Society for Diabetes in Childhood and Adolescence. Residual beta-Cell function 3-6 years after onset of type 1 diabetes reduces risk of severe hypoglycemia in children and adolescents. Diabetes Care. 2013;36:3454–3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steffes MW, Sibley S, Jackson M, et al. β-cell function and the development of diabetes-related complications in the diabetes control and complications trial. Diabetes Care. 2003;26:832–836. [DOI] [PubMed] [Google Scholar]
- 36.McEachron KR, Yang Y, Hodges JS, et al. Performance of modified Igls criteria to evaluate islet autograft function after total pancreatectomy with islet autotransplantation—a retrospective study. Transpl Int. 2021;34:87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gołębiewska JE, Bachul PJ, Fillman N, et al. Assessment of simple indices based on a single fasting blood sample as a tool to estimate beta-cell function after total pancreatectomy with islet autotransplantation–a prospective study. Transpl Int. 2019;32:280–290. [DOI] [PubMed] [Google Scholar]
- 38.Pollard CA, Chung WY, Garcea G, et al. Assessment of long-term graft function following total pancreatectomy and autologous islet transplantation: the Leicester experience. Hepatob Surg Nutrition. 2022;12:682–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tai DS, Shen N, Szot GL, et al. Autologous islet transplantation with remote islet isolation after pancreas resection for chronic pancreatitis. JAMA surgery. 2015;150:118–124. [DOI] [PubMed] [Google Scholar]
- 40.Kesseli SJ, Wagar M, Jung MK, et al. Long-term glycemic control in adult patients undergoing remote vs. local total pancreatectomy with islet autotransplantation. Am J Gastroenterol. 2017;112:643–649. [DOI] [PubMed] [Google Scholar]
- 41.Sugiura T, Uesaka K, Mihara K, et al. Margin status, recurrence pattern, and prognosis after resection of pancreatic cancer. Surgery. 2013;154:1078–1086. [DOI] [PubMed] [Google Scholar]
- 42.Daamen LA, Groot VP, Besselink MG, et al. ; Dutch Pancreatic Cancer Group. Detection, treatment, and survival of pancreatic cancer recurrence in the Netherlands: a nationwide analysis. Ann Surg. 2022;275:769–775. [DOI] [PubMed] [Google Scholar]
- 43.Tamburrino D, Partelli S, Crippa S, et al. Selection criteria in resectable pancreatic cancer: a biological and morphological approach. World J Gastroenterol. 2014;20:11210–11215. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.