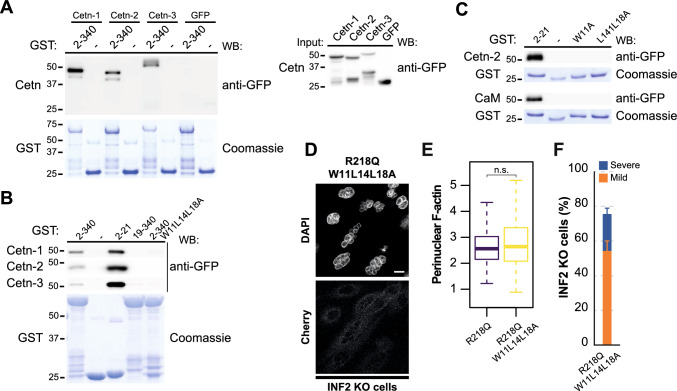
Fig. 6.
The formation of nuclear abnormalities is independent of centrin or calmodulin binding to pathogenic INF2. A GST fused to the 2-340 INF2 fragment (GST-INF2 2-340), which encompasses the INF2 DID, was used in pull-down experiments with GFP-centrin (Cetn)-1, -2 and -3 or GFP alone (left panel). The input of the four GFP proteins used is shown (right panel). B Pull-down of various INF2 fragments (2-340, 2-21, 19-340, and 2-340 with the W11L14L18A mutation) with GFP-Cent-1, -2 and -3. C Pull-down of the 2-21 INF2 fragment with the indicated mutations in residues W11, L14 and L18 with GFP-Cent-2 or GFP-CaM. Purified GST proteins were stained with Coomassie blue to control for the amount of GST protein used in (A–C). D Image of an equatorial plane of INF2 KO MDCK cells expressing INF2 R218Q W11L14L18A. Nuclei were visualized with DAPI. Scale bar, 10 μm. E Perinuclear F-actin levels in INF2 R218Q and INF2 R218Q W11L14L18A cells. More than 150 cells were examined; three independent experiments. F Percentage of INF2 R218Q W11L14L18A cells displaying an abnormal nuclear phenotype. More than 300 cells were analyzed; three independent experiments. n.s., not significant