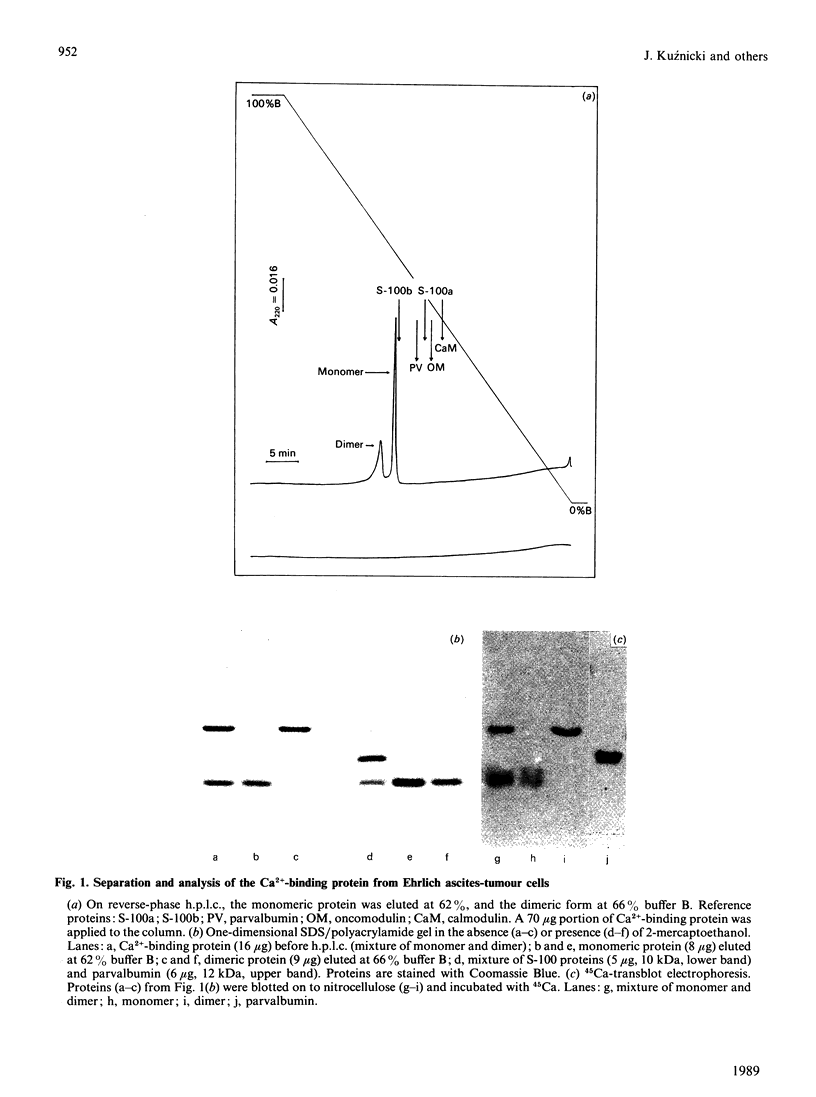
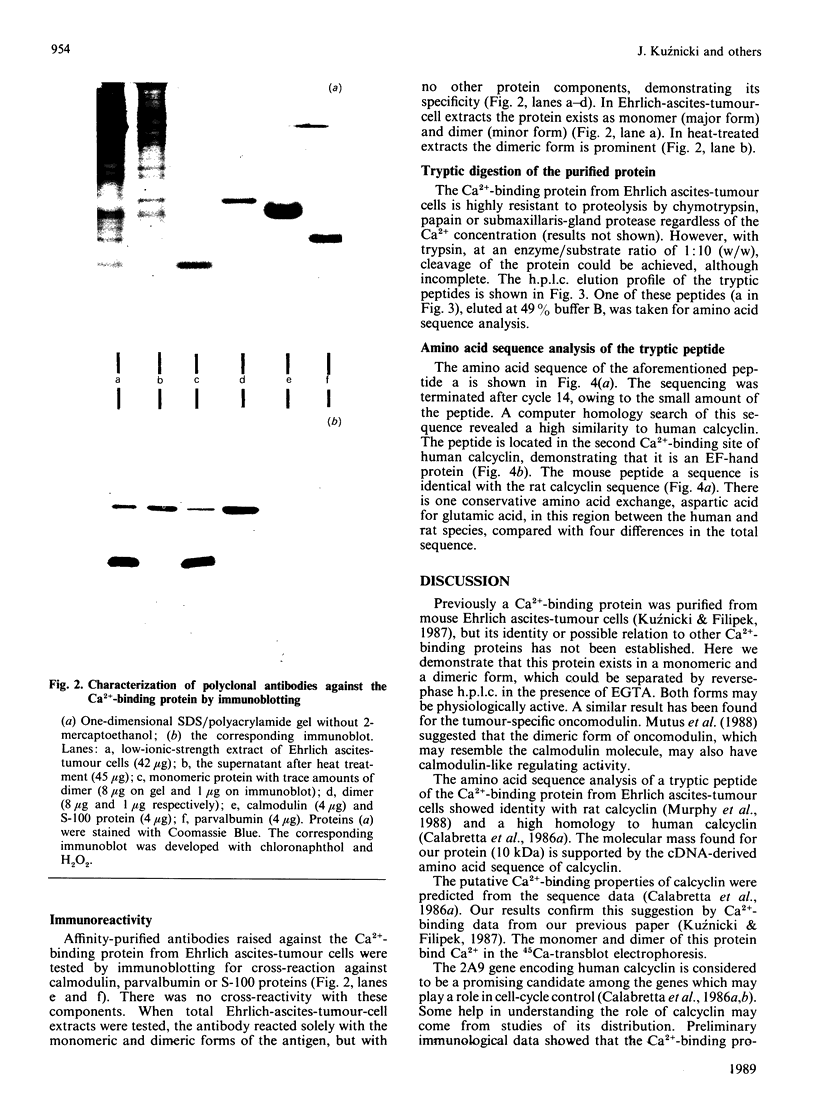
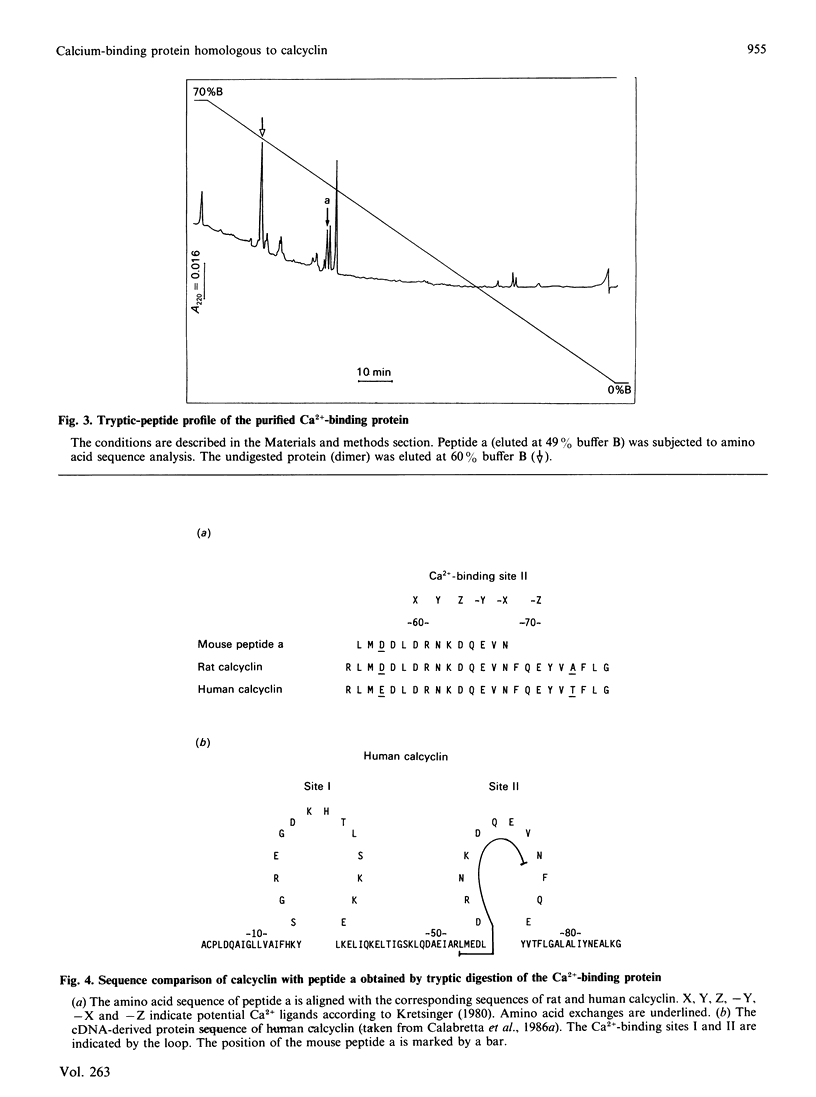
Abstract
A Ca2+-binding protein was purified from mouse Ehrlich ascites-tumour cells. The protein forms monomers and disulphide-linked dimers, which can be separated by reverse-phase h.p.l.c. A partial amino acid sequence analysis demonstrated that the protein has an EF-hand structure. A striking homology was found to rat and human calcyclin (a member of the S-100 protein family), which is possibly involved in cell-cycle regulation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berchtold M. W., Wilson K. J., Heizmann C. W. Isolation of neuronal parvalbumin by high-performance liquid chromatography. Characterization and comparison with muscle parvalbumin. Biochemistry. 1982 Dec 7;21(25):6552–6557. doi: 10.1021/bi00268a035. [DOI] [PubMed] [Google Scholar]
- Calabretta B., Battini R., Kaczmarek L., de Riel J. K., Baserga R. Molecular cloning of the cDNA for a growth factor-inducible gene with strong homology to S-100, a calcium-binding protein. J Biol Chem. 1986 Sep 25;261(27):12628–12632. [PubMed] [Google Scholar]
- Calabretta B., Venturelli D., Kaczmarek L., Narni F., Talpaz M., Anderson B., Beran M., Baserga R. Altered expression of G1-specific genes in human malignant myeloid cells. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1495–1498. doi: 10.1073/pnas.83.5.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drabikowski W., Kuznicki J., Grabarek Z. Similarity in Ca2+-induced changes between troponic-C and protein activator of 3':5'-cyclic nucleotide phosphodiesterase and their tryptic fragments. Biochim Biophys Acta. 1977 Nov 23;485(1):124–133. doi: 10.1016/0005-2744(77)90199-1. [DOI] [PubMed] [Google Scholar]
- Ferrari S., Calabretta B., deRiel J. K., Battini R., Ghezzo F., Lauret E., Griffin C., Emanuel B. S., Gurrieri F., Baserga R. Structural and functional analysis of a growth-regulated gene, the human calcyclin. J Biol Chem. 1987 Jun 15;262(17):8325–8332. [PubMed] [Google Scholar]
- Heizmann C. W. Parvalbumin, an intracellular calcium-binding protein; distribution, properties and possible roles in mammalian cells. Experientia. 1984 Sep 15;40(9):910–921. doi: 10.1007/BF01946439. [DOI] [PubMed] [Google Scholar]
- Kligman D., Hilt D. C. The S100 protein family. Trends Biochem Sci. 1988 Nov;13(11):437–443. doi: 10.1016/0968-0004(88)90218-6. [DOI] [PubMed] [Google Scholar]
- Kretsinger R. H. Structure and evolution of calcium-modulated proteins. CRC Crit Rev Biochem. 1980;8(2):119–174. doi: 10.3109/10409238009105467. [DOI] [PubMed] [Google Scholar]
- Kuźnicki J., Filipek A. Purification and properties of a novel Ca2+-binding protein (10.5 kDa) from Ehrlich-ascites-tumour cells. Biochem J. 1987 Nov 1;247(3):663–667. doi: 10.1042/bj2470663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marshak D. R., Watterson D. M., Van Eldik L. J. Calcium-dependent interaction of S100b, troponin C, and calmodulin with an immobilized phenothiazine. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6793–6797. doi: 10.1073/pnas.78.11.6793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama K., Mikawa T., Ebashi S. Detection of calcium binding proteins by 45Ca autoradiography on nitrocellulose membrane after sodium dodecyl sulfate gel electrophoresis. J Biochem. 1984 Feb;95(2):511–519. doi: 10.1093/oxfordjournals.jbchem.a134633. [DOI] [PubMed] [Google Scholar]
- Murphy L. C., Murphy L. J., Tsuyuki D., Duckworth M. L., Shiu R. P. Cloning and characterization of a cDNA encoding a highly conserved, putative calcium binding protein, identified by an anti-prolactin receptor antiserum. J Biol Chem. 1988 Feb 15;263(5):2397–2401. [PubMed] [Google Scholar]
- Mutus B., Palmer E. J., MacManus J. P. Disulfide-linked dimer of oncomodulin: comparison to calmodulin. Biochemistry. 1988 Jul 26;27(15):5615–5622. doi: 10.1021/bi00415a033. [DOI] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]