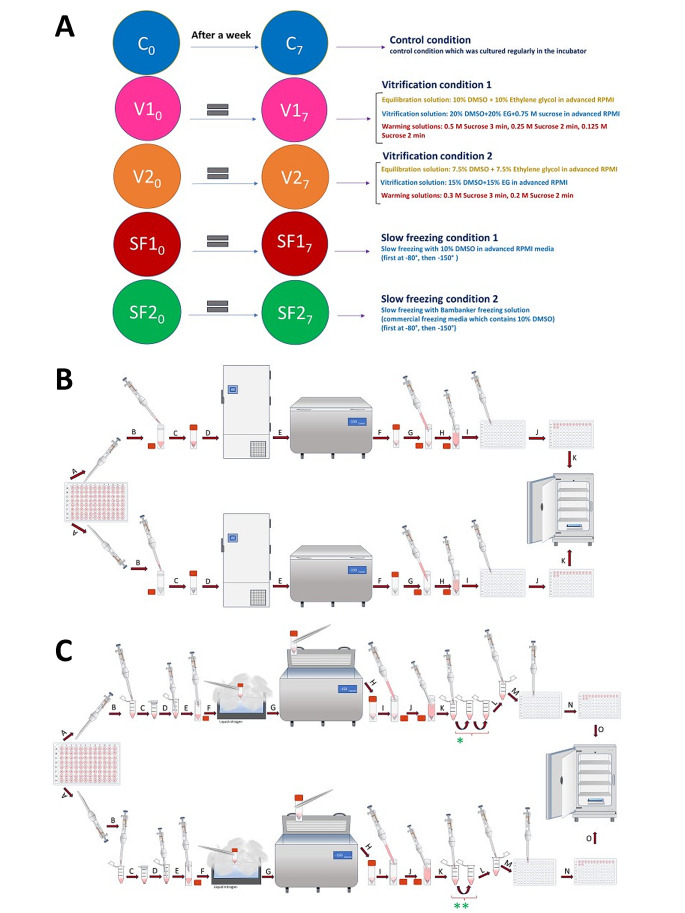
Fig. 9.
Illustrating the experimental conditions and methodologies utilized for different freezing techniques in this study. (A) This study incorporated five distinct experimental conditions, delineating the specifics of each component. All replicates originated from human stem cells, with the kidney organoids being of BJFF type. (B) In-depth delineation of slow freezing techniques. The upper section pertains to Slow Freezing number one (SF1), while the lower section pertains to Slow Freezing number two (SF2). All procedural steps remained identical, barring the variation in freezing solutions utilized in this experimental setup. Providing comprehensive elaboration on the sequential steps involved: A- Extracting four organoids using the pipette at its lowest power to minimize media volume. B- Transferring replicates into cryotubes containing 200 μl of freezing media; the upper cryotube (SF1) contains 10% DMSO and advanced RPMI with glutamax, while the lower one (SF2) contains commercial freezing media (Bambanker). C- Ensuring the proper closure of cryotube caps. D- Placing cryotubes in a -80 degree freezer for 24 h. E- Moving cryotubes to a -150-degree freezer for an additional 6 days. F - Retrieving cryotubes after 7 days of freezing for thawing. G - Adding 1 milliliter of fresh room-temperature media for thawing. H - Gently extract replicates using the pipette at the lowest possible power. I - Transferring them into new 96-well plates. J - Moving all 14 replicates of each condition onto the same plate. K - Placing plates into the incubator for regular growth. (C) Detailed explanation of ultra-rapid freezing (Vitrification) techniques. The upper segment denotes Vitrification number one (V1), while the lower segment pertains to Vitrification number two (V2). All steps within the process remained consistent, differing solely in the equilibration, vitrification, and warming solutions applied in this experimental setup. A-Extracting four organoids using the pipette at minimal power to minimize media volume. B-Transferring replicates into Eppendorf tubes containing 200 μl of equilibration solution. The upper tube (ES1) comprises 10% DMSO, 10% Ethylene glycol in advanced RPMI with glutamax, and the lower tube (ES2) holds 7.5% DMSO, 7.5% Ethylene glycol in advanced RPMI with glutamax. C-Immerse the organoids into an equilibration solution at room temperature, with exposure times averaging 8–15 min to allow penetration of cryopreserved agents into the kidney organoids. D-Removing replicates to transfer them into specific vitrification solutions. E- Placing replicates into the vitrification solutions; each containing 200 μl. Vitrification solution 1 (VS1) comprises 20% DMSO, 20% Ethylene glycol in advanced RPMI with glutamax, while Vitrification solution 2 (VS2) includes 15% DMSO, 15% Ethylene glycol in advanced RPMI with glutamax. Exposure time should not exceed 30 s due to high toxicity; this process took less than 10 s in this experiment. F- Directly exposing closed-cap cryotubes to liquid nitrogen for 10 s. G- Placing cryotubes directly into the − 150-degree freezer for 7 days. H- Retrieving cryotubes after 7 days of freezing. I- Adding 1 milliliter of fresh room-temperature RPMI media for thawing. J- Removing replicates for multi-step warming solutions. K- Placing organoids into specific warming solutions; each containing 200 μl. *- V1 warming involves 3 steps: 0.5 M sucrose in advanced RPMI with glutamax for 3 min, followed by 0.25 M sucrose for 2 min, and lastly, 0.125 M sucrose for 2 min. **- V2 warming entails 2 steps: 0.3 M sucrose for 3 min then 0.2 M sucrose for 2 min in advanced RPMI with glutamax. L- Retrieving replicates for placement into new 96-well plates. M- Transferring them into the new 96-well plate. N- Moving all 14 organoids of each condition into the same plate. O- Placing the plates into the incubator for regular growth