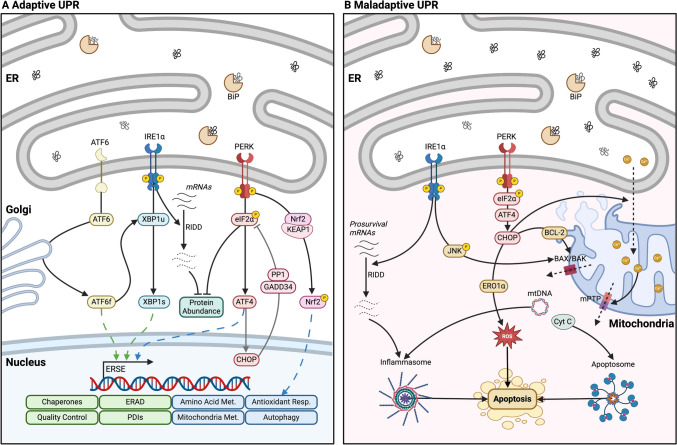
Fig. 1.
The UPRER. A Adaptive UPRER. Following ER stress, BiP binds to misfolded proteins on the substrate-binding site and the ATPase domain dissociates from the transmembrane receptors, allowing allosteric activation of the UPRER regulators by oligomerisation and phosphorylation [14, 15]. (1) IRE1α RNase activity mediates unconventional splicing of XBP1 [16–18], XBP1s translocates to the nucleus to promote expression of genes related to quality control [9, 19]. IRE1α also mediates the cleavage and degradation of mRNAs and microRNAs; regulated IRE1α-dependent decay (RIDD), decreasing the protein load in the ER lumen [20]. (2) PERK phosphorylates eIF2α [21], promoting rapid attenuation of global mRNA translation [22, 23]. Phosphorylated eIF2α also regulates the translation of the transcription factor ATF4 [24]. ATF4 regulates the feedback loop responsible for the restoration of protein synthesis. ATF4 induction of CHOP, upregulates the expression of GADD34 which forms a complex with PP1 to dephosphorylate eIF2α [25, 26]. (3) ATF6α translocates to the Golgi apparatus, where it is cleaved to generate ATF6f, which acts as a transcription factor that promotes the expression of ER chaperones [27, 28]. ATF6α promotes the expression of Xbp1 mRNA, enhancing the substrate load for IRE1α splicing [29]. B Maladaptive UPRER. Following prolonged ER stress the homeostatic capacity of the UPRER becomes saturated that can activate pro-apoptotic signalling. (1) IRE1α interacts with TRAF2 to promote a kinase signalling cascade that activates JNK [30, 31]. JNK promotes the oligomerisation of BAX and BAK on the mitochondrial membrane and the assembly of the apoptosome [32, 33]. RIDD can promote apoptosis by degrading essential cell-survival mRNAs such as the negative regulators of TXNIP, promoting the assembly of the inflammasome leading to apoptosis [34, 35]. (2) PERK-eIF2α induces the translation of ATF4, activation of CHOP and GADD34 [25, 26]. CHOP promotes the expression of PUMA, NOXA, BIM and BID, which induce the mitochondrial BCL-2 pro-apoptotic proteins. CHOP can also activate the translation of ERO1α, promoting the oxidation of the ER environment [36, 37]. PERK-ATF4-CHOP arm regulates IP3R-mediated Ca2+ leakage from the ER [38, 39]. Sustained and excessive Ca2+ transport from the ER to the mitochondria impairs mitochondrial metabolism and lead to opening of the mPTP and pro-apoptotic signalling [40, 41]