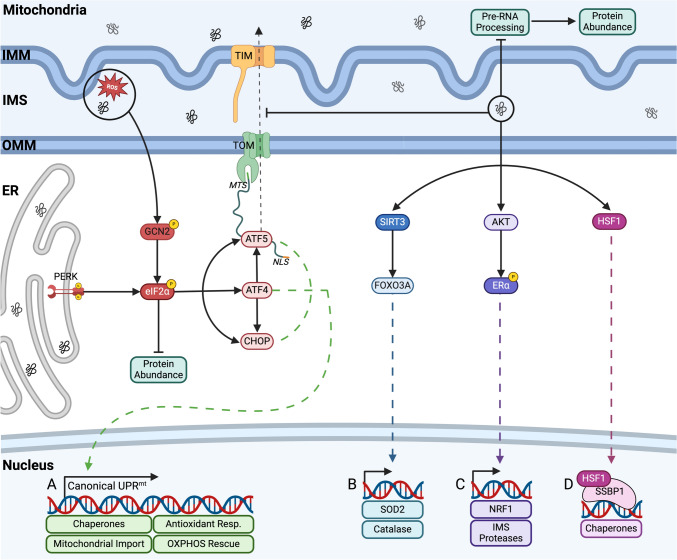
Fig. 2.
The UPRmt. Most proteins that constitute the mitochondrial proteome are synthesised in the cytoplasm, targeted and imported into mitochondria [125] via the TOM/TIM complex [126], perturbation of this trafficking can impair mitochondrial proteostasis and induce mitochondrial stress [127]. A The canonical axis of the UPRmt is controlled by the expression of ATF5, ATF4 and CHOP [132]. CHOP alleviates proteotoxic stress by inducing the expression of the mitochondrial chaperones HSP10 and HSP60 [134]. ATF5 is normally imported into mitochondria via TOM and TIM, where it is degraded by proteases [138]. Mitochondrial proteotoxic stress will perturb mitochondrial import efficiency, resulting in the activation of ATF5 by p-eIF2α and its translocation to the nucleus [139]. ATF5 promotes the transcription of genes related to chaperones, proteases and antioxidant proteins [137]. B The sirtuin axis of the UPRmt activates SIRT3, which deacetylates FOXO3A, promoting its translocation to the nucleus and transcription of SOD2 and catalase [142, 143]. C AKT mediates the ROS-dependant phosphorylation of ERα, which activates NRF1 and the IMS protease HTRA2 [144]. NRF1 stimulates mitochondrial respiration, proteasome activity and the IMS protease OMI. D Mitochondrial proteotoxic stress promotes epigenetic changes in the cellular DNA regulated by HSF1, it translocates to the nucleus where it interacts with SSBP1 to bind to the chromatin and boost the expression of mitochondrial chaperones [145, 146]