Abstract
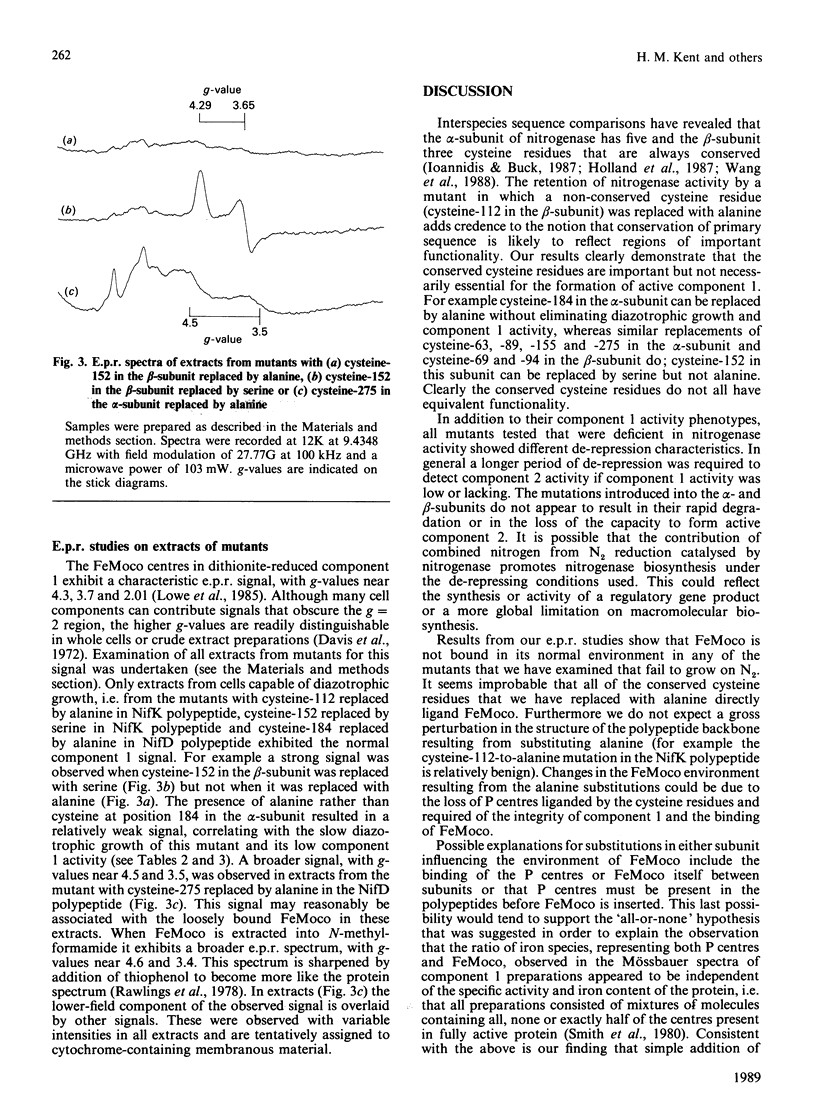
The five conserved cysteine residues present in the alpha-subunit and the three conserved cysteine residues present in the beta-subunit of nitrogenase component 1 were individually changed to alanine. Mutations in the alpha-subunit at positions 63, 89, 155 and 275 and in the beta-subunit at positions 69, 94 and 152 all resulted in a loss of diazotrophic growth and component 1 activity and loss of the normal e.p.r. signal of the component 1 protein. Component 2 activity was retained. Replacement of cysteine-184 in the alpha-subunit with alanine greatly diminished, but did not eliminate, diazotrophic growth and component 1 activity. Substitution of serine for cysteine at position 152 in the beta-subunit, in contrast with the substitution of alanine at this position, resulted in the formation of active component 1. Replacement of the non-conserved cysteine-112 in the beta-subunit with alanine did not greatly perturb diazotrophic growth or the activity of component 1. Extracts prepared from a mutant, with cysteine-275 of the alpha-subunit replaced by alanine, complemented extracts of a mutant unable to synthesize the iron-molybdenum cofactor of nitrogenase, indicating that the alanine-275 substitution increases the availability of cofactor. Furthermore extracts of this mutant exhibited an e.p.r. signal similar to that of extracted iron-molybdenum cofactor. These data suggest a role for cysteine-275 as a ligand to the cofactor.
Full text
PDF

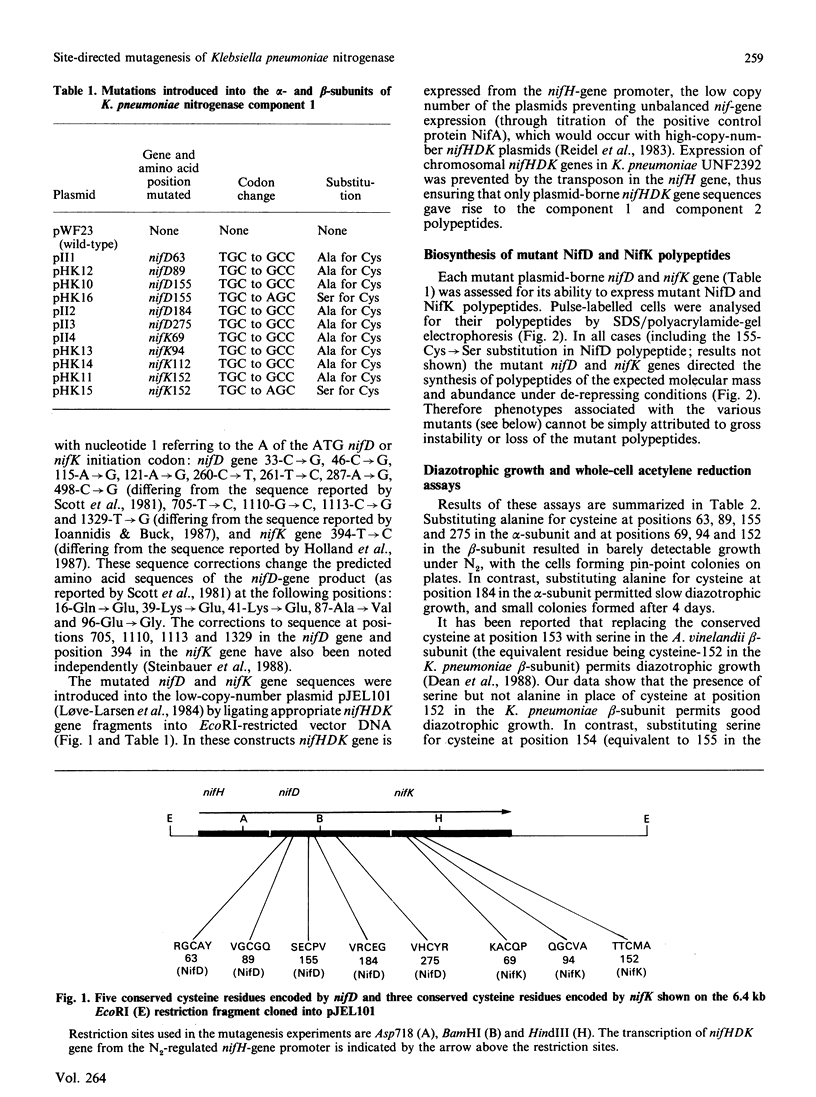
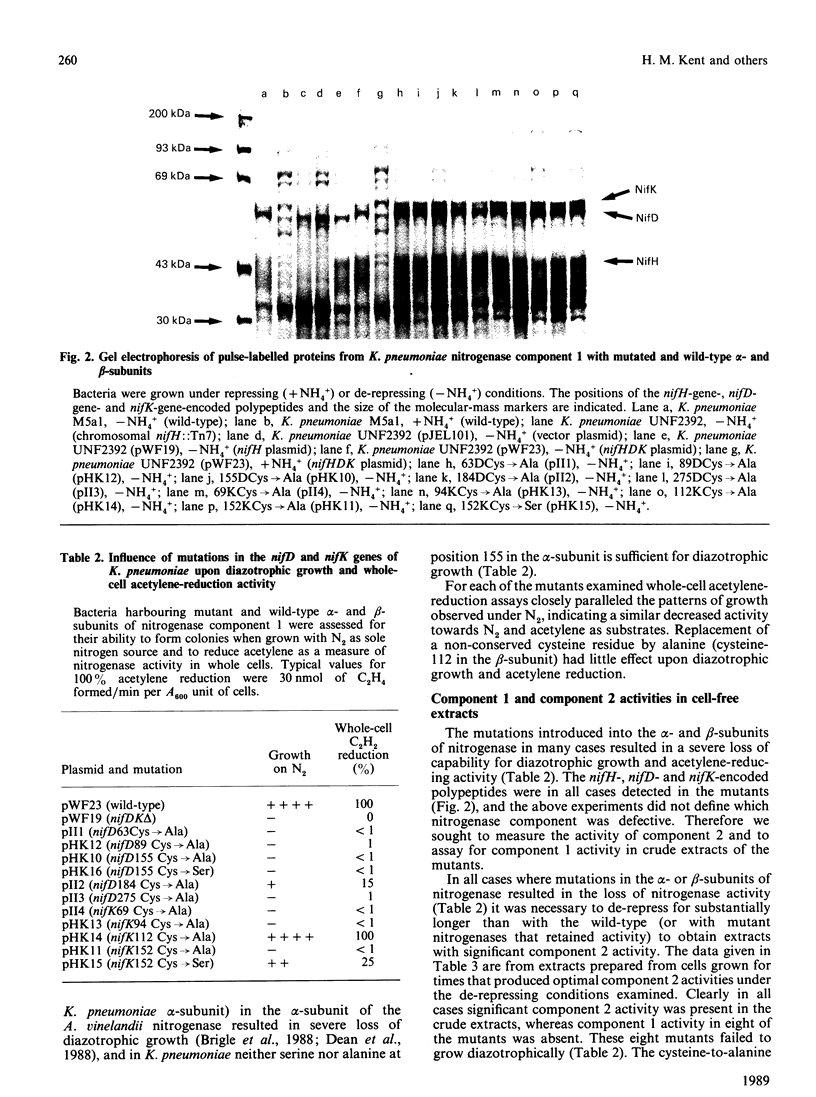
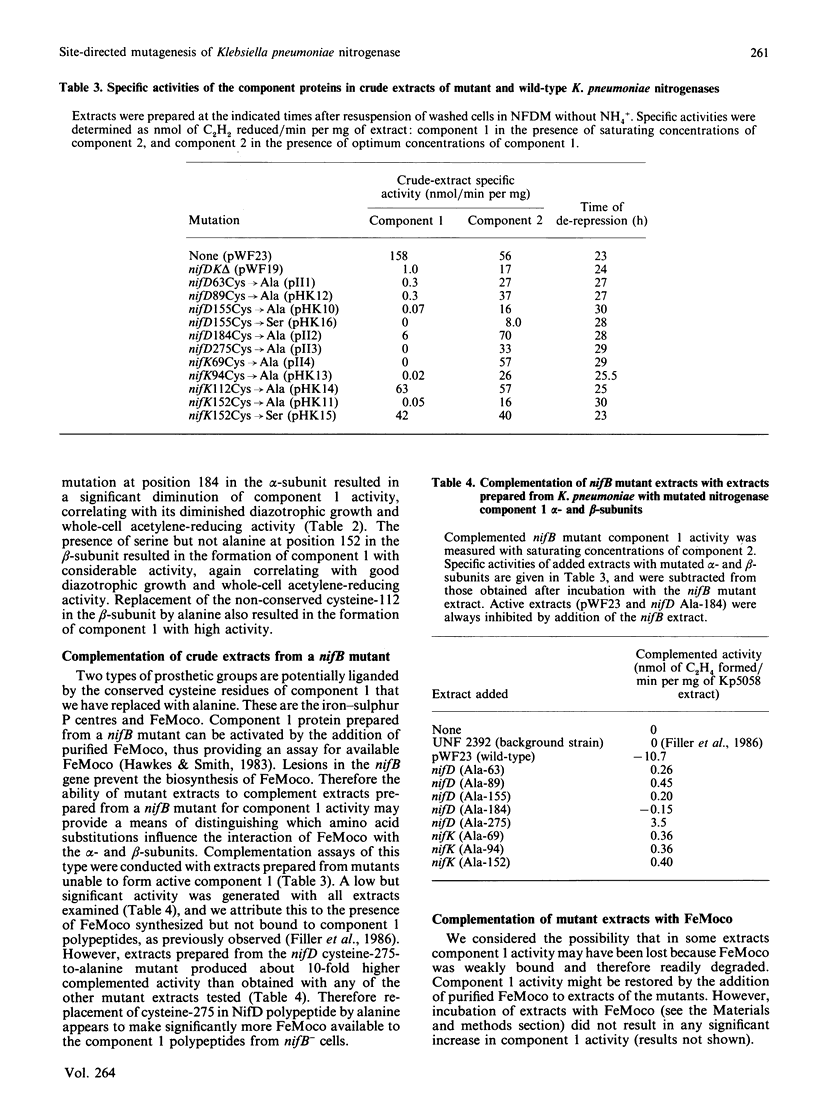


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brigle K. E., Newton W. E., Dean D. R. Complete nucleotide sequence of the Azotobacter vinelandii nitrogenase structural gene cluster. Gene. 1985;37(1-3):37–44. doi: 10.1016/0378-1119(85)90255-0. [DOI] [PubMed] [Google Scholar]
- Brigle K. E., Setterquist R. A., Dean D. R., Cantwell J. S., Weiss M. C., Newton W. E. Site-directed mutagenesis of the nitrogenase MoFe protein of Azotobacter vinelandii. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7066–7069. doi: 10.1073/pnas.84.20.7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck M., Cannon W., Woodcock J. Mutational analysis of upstream sequences required for transcriptional activation of the Klebsiella pneumoniae nifH promoter. Nucleic Acids Res. 1987 Dec 10;15(23):9945–9956. doi: 10.1093/nar/15.23.9945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck M., Khan H., Dixon R. Site-directed mutagenesis of the Klebsiella pneumoniae nifL and nifH promoters and in vivo analysis of promoter activity. Nucleic Acids Res. 1985 Nov 11;13(21):7621–7638. doi: 10.1093/nar/13.21.7621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess B. K., Stiefel E. I., Newton W. E. Oxidation-reduction properties and complexation reactions of the iron-molybdenum cofactor of nitrogenase. J Biol Chem. 1980 Jan 25;255(2):353–356. [PubMed] [Google Scholar]
- Cannon F. C., Dixon R. A., Postgate J. R., Primrose S. B. Chromosomal integration of Klebsiella nitrogen fixation genes in Escherichia coli. J Gen Microbiol. 1974 Jan;80(1):227–239. doi: 10.1099/00221287-80-1-227. [DOI] [PubMed] [Google Scholar]
- Cannon F. C., Riedel G. E., Ausubel F. M. Recombinant plasmid that carries part of the nitrogen fixation (nif) gene cluster of Klebsiella pneumoniae. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2963–2967. doi: 10.1073/pnas.74.7.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis L. C., Shah V. K., Brill W. J., Orme-Johnson W. H. Nitrogenase. II. Changes in the EPR signal of component I (iron-molybdenum protein) of Azotobacter vinelandii nitrogenase during repression and derepression. Biochim Biophys Acta. 1972 Feb 28;256(2):512–523. doi: 10.1016/0005-2728(72)90079-5. [DOI] [PubMed] [Google Scholar]
- Eady R. R., Smith B. E., Cook K. A., Postgate J. R. Nitrogenase of Klebsiella pneumoniae. Purification and properties of the component proteins. Biochem J. 1972 Jul;128(3):655–675. doi: 10.1042/bj1280655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filler W. A., Kemp R. M., Ng J. C., Hawkes T. R., Dixon R. A., Smith B. E. The nifH gene product is required for the synthesis or stability of the iron-molybdenum cofactor of nitrogenase from Klebsiella pneumoniae. Eur J Biochem. 1986 Oct 15;160(2):371–377. doi: 10.1111/j.1432-1033.1986.tb09981.x. [DOI] [PubMed] [Google Scholar]
- Hagen W. R., Wassink H., Eady R. R., Smith B. E., Haaker H. Quantitative EPR of an S = 7/2 system in thionine-oxidized MoFe proteins of nitrogenase. A redefinition of the P-cluster concept. Eur J Biochem. 1987 Dec 15;169(3):457–465. doi: 10.1111/j.1432-1033.1987.tb13633.x. [DOI] [PubMed] [Google Scholar]
- Hawkes T. R., McLean P. A., Smith B. E. Nitrogenase from nifV mutants of Klebsiella pneumoniae contains an altered form of the iron-molybdenum cofactor. Biochem J. 1984 Jan 1;217(1):317–321. doi: 10.1042/bj2170317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes T. R., Smith B. E. Purification and characterization of the inactive MoFe protein (NifB-Kp1) of the nitrogenase from nifB mutants of Klebsiella pneumoniae. Biochem J. 1983 Jan 1;209(1):43–50. doi: 10.1042/bj2090043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland D., Zilberstein A., Zamir A., Sussman J. L. A quantitative approach to sequence comparisons of nitrogenase MoFe protein alpha- and beta-subunits including the newly sequenced nifK gene from Klebsiella pneumoniae. Biochem J. 1987 Oct 15;247(2):277–285. doi: 10.1042/bj2470277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannidis I., Buck M. Nucleotide sequence of the Klebsiella pneumoniae nifD gene and predicted amino acid sequence of the alpha-subunit of nitrogenase MoFe protein. Biochem J. 1987 Oct 15;247(2):287–291. doi: 10.1042/bj2470287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz D. M., Jr, McMillan R. S., Burgess B. K., Mortenson L. E., Holm R. H. Identification of iron-sulfur centers in the iron-molybdenum proteins of nitrogenase. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4986–4989. doi: 10.1073/pnas.76.10.4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larsen J. E., Gerdes K., Light J., Molin S. Low-copy-number plasmid-cloning vectors amplifiable by derepression of an inserted foreign promoter. Gene. 1984 Apr;28(1):45–54. doi: 10.1016/0378-1119(84)90086-6. [DOI] [PubMed] [Google Scholar]
- McLean P. A., Papaefthymiou V., Orme-Johnson W. H., Münck E. Isotopic hybrids of nitrogenase. Mössbauer study of MoFe protein with selective 57Fe enrichment of the P-cluster. J Biol Chem. 1987 Sep 25;262(27):12900–12903. [PubMed] [Google Scholar]
- Riedel G. E., Brown S. E., Ausubel F. M. Nitrogen fixation by Klebsiella pneumoniae is inhibited by certain multicopy hybrid nif plasmids. J Bacteriol. 1983 Jan;153(1):45–56. doi: 10.1128/jb.153.1.45-56.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott K. F., Rolfe B. G., Shine J. Biological nitrogen fixation: primary structure of the Klebsiella pneumoniae nifH and nifD genes. J Mol Appl Genet. 1981;1(1):71–81. [PubMed] [Google Scholar]
- Shah V. K., Brill W. J. Isolation of an iron-molybdenum cofactor from nitrogenase. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3249–3253. doi: 10.1073/pnas.74.8.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B. E., O'Donnell M. J., Lang G., Spartalian K. A Mössbauer spectroscopic investigation of the redox behaviour of the molybdenum-iron protein from Klebsiella pneumoniae nitrogenase. Mechanistic and structural implications. Biochem J. 1980 Nov 1;191(2):449–455. doi: 10.1042/bj1910449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbauer J., Wenzel W., Hess D. Nucleotide and deduced amino acid sequences of the Klebsiella pneumoniae nifK gene coding for the beta-subunit of nitrogenase MoFe protein. Nucleic Acids Res. 1988 Jul 25;16(14B):7199–7199. doi: 10.1093/nar/16.14.7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. Z., Chen J. S., Johnson J. L. Distinct structural features of the alpha and beta subunits of nitrogenase molybdenum-iron protein of Clostridium pasteurianum: an analysis of amino acid sequences. Biochemistry. 1988 Apr 19;27(8):2800–2810. doi: 10.1021/bi00408a021. [DOI] [PubMed] [Google Scholar]
- Zimmermann R., Münck E., Brill W. J., Shah V. K., Henzl M. T., Rawlings J., Orme-Johnson W. H. Nitrogenase X: Mössbauer and EPR studies on reversibly oxidized MoFe protein from Azotobacter vinelandii OP. Nature of the iron centers. Biochim Biophys Acta. 1978 Dec 20;537(2):185–207. doi: 10.1016/0005-2795(78)90504-4. [DOI] [PubMed] [Google Scholar]



