Abstract
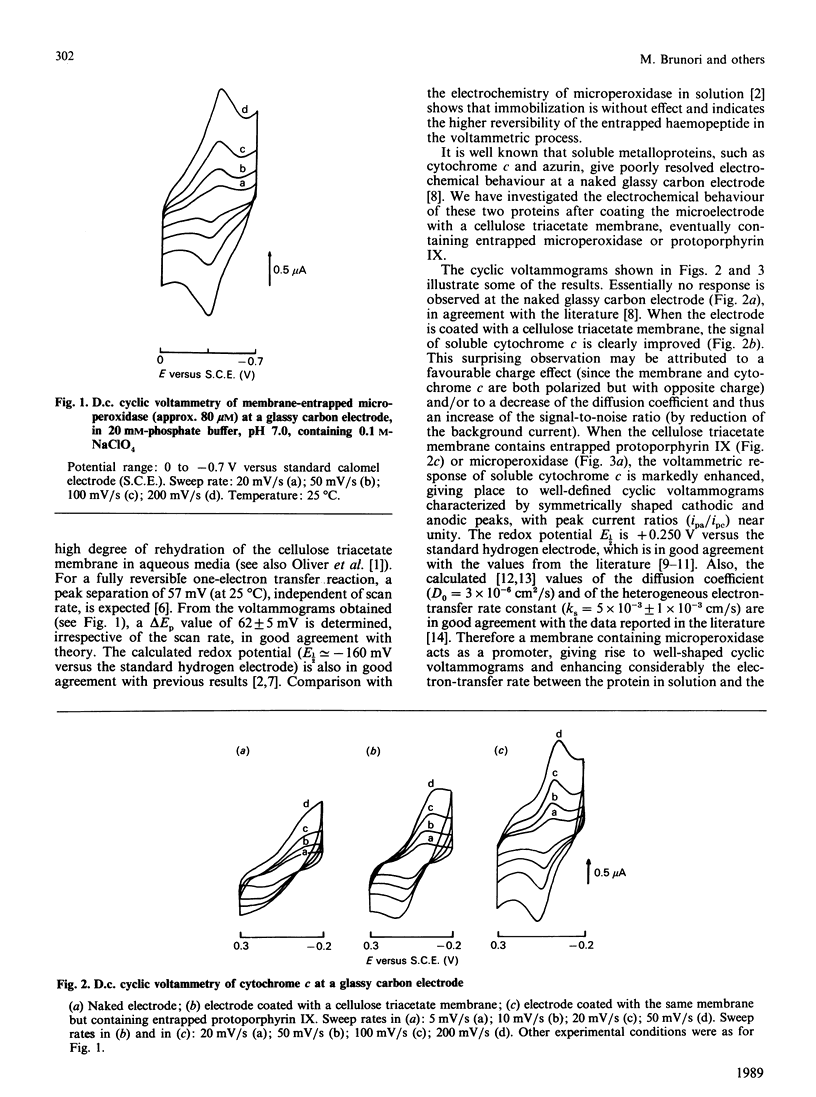
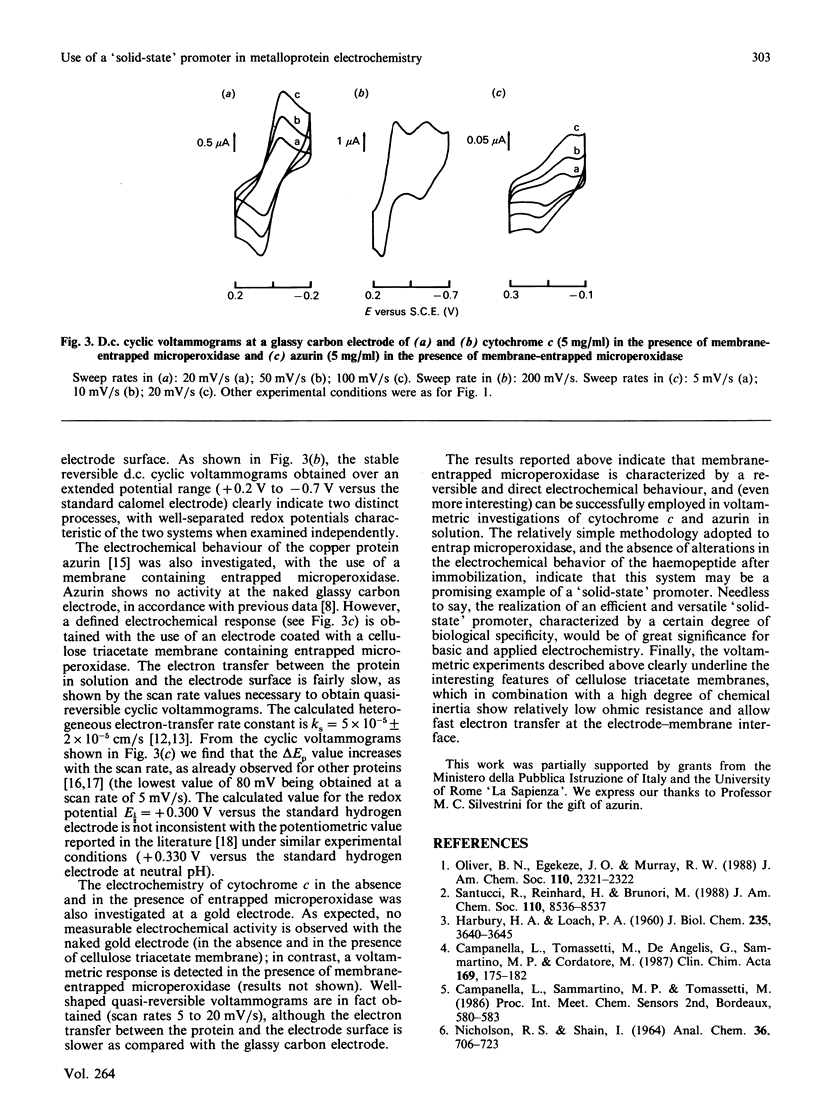
Immobilization of biological systems in solid matrices is presently of great interest, in view of the many potential advantages associated with both the higher stability of the immobilized macromolecules and the potential utilization for biotechnology. In the present paper the electrochemical behaviour of the undecapeptide from cytochrome c (called microperoxidase) tightly entrapped in cellulose triacetate membrane is reported; its utilization as 'solid-state' promoter in the electrochemistry of soluble metalloproteins is presented. The results obtained indicate that: (i) membrane-entrapped microperoxidase undergoes rapid reversible electron transfer at a glassy carbon electrode; (ii) the electrochemical process is diffusion-controlled; (iii) entrapped microperoxidase acts as 'solid-state' promoter in the electrochemistry of soluble cytochrome c and of azurin.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMBLER R. P. THE PURIFICATION AND AMINO ACID COMPOSITION OF PSEUDOMONAS CYTOCHROME C-551. Biochem J. 1963 Nov;89:341–349. doi: 10.1042/bj0890341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong F. A., Allen H., Hill O., Walton N. J. Reactions of electron-transfer proteins at electrodes. Q Rev Biophys. 1985 Aug;18(3):261–322. doi: 10.1017/s0033583500000366. [DOI] [PubMed] [Google Scholar]
- Campanella L., Tomassetti M., De Angelis G., Sammartino M. P., Cordatore M. A new assay for choline-containing phospholipids in amniotic fluid by an enzyme sensor. Clin Chim Acta. 1987 Nov 16;169(2-3):175–182. doi: 10.1016/0009-8981(87)90317-2. [DOI] [PubMed] [Google Scholar]
- HARBURY H. A., LOACH P. A. Oxidation-linked proton functions in heme octa- and undecapeptides from mammalian cytochrome c. J Biol Chem. 1960 Dec;235:3640–3645. [PubMed] [Google Scholar]
- HENDERSON R. W., RAWLINSON W. A. Potentiometric and other studies on preparations of cytochrome c from ox- and horse-heart muscle. Biochem J. 1956 Jan;62(1):21–29. doi: 10.1042/bj0620021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbury H. A., Loach P. A. LINKED FUNCTIONS IN HEME SYSTEMS: OXIDATION-REDUCTION POTENTIALS AND ABSORPTION SPECTRA OF A HEME PEPTIDE OBTAINED UPON PEPTIC HYDROLYSIS OF CYTOCHROME C. Proc Natl Acad Sci U S A. 1959 Sep;45(9):1344–1359. doi: 10.1073/pnas.45.9.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkridge F. M., Kuwana T. Indirect coulometric titration of biological electron transport components. Anal Chem. 1973 Jun;46(7):1021–1026. doi: 10.1021/ac60329a038. [DOI] [PubMed] [Google Scholar]


