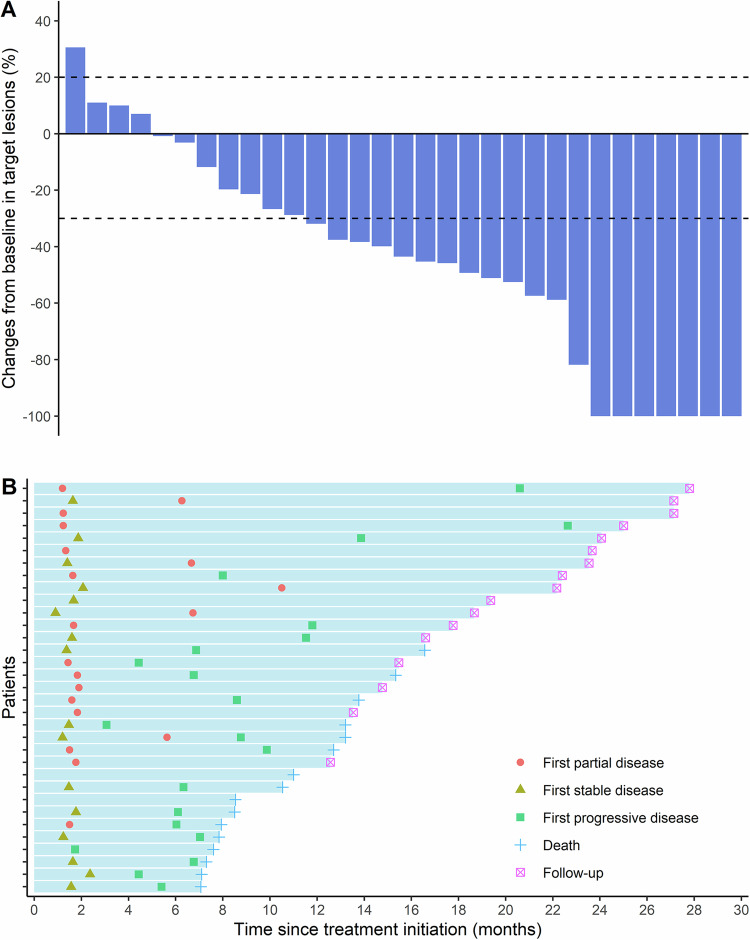
Fig. 3. Tumor responses.
A Best percentage changes in target lesion sizes from baseline (n = 31). Dashed lines at +20% and −30% represent thresholds for disease progression and partial response, respectively, according to the RECIST 1.1 criteria. Among the total 33 patients, One patient withdrew informed consent after one cycle of chemo-immunotherapy. One patient experienced severe cardiac adverse events after two cycles of chemo-immunotherapy. Both patients refused further assessment. Therefore, only 31 patients had a baseline and at least one post-baseline radiologic assessment. B Onset of response, duration of response, and outcome (n = 33). Source data are provided as a Source Data file. RECIST Response Evaluation Criteria in Solid Tumors.