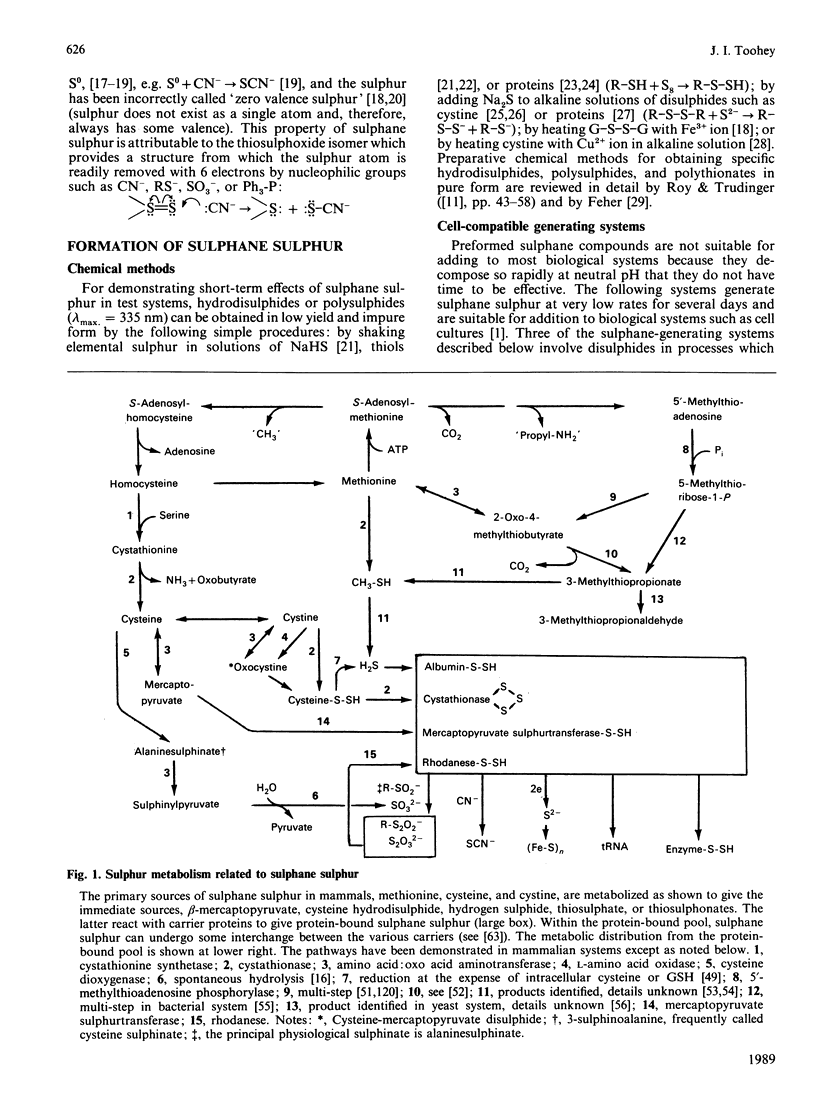
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agris P. F., Armstrong D. J., Schäfer K. P., Söll D. Maturation of a hypermodified nucleoside in transfer RNA. Nucleic Acids Res. 1975 May;2(5):691–698. doi: 10.1093/nar/2.5.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrò A. F., Mavelli I., Cannella C., Federici G. Activation of porcine heart mitochondrial malate dehydrogenase by zero valence sulfur and rhodanese. Biochem Biophys Res Commun. 1976 Jan 26;68(2):553–560. doi: 10.1016/0006-291x(76)91181-5. [DOI] [PubMed] [Google Scholar]
- Ajitkumar P., Cherayil J. D. Thionucleosides in transfer ribonucleic acid: diversity, structure, biosynthesis, and function. Microbiol Rev. 1988 Mar;52(1):103–113. doi: 10.1128/mr.52.1.103-113.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apffel C. A., Walker J. E., Issarescu S. Tumor rejection in experimental animals treated with radioprotective thiols. Cancer Res. 1975 Feb;35(2):429–437. [PubMed] [Google Scholar]
- Apple M. A., Greenberg D. M. Inhibitory effect of DL-2-mercapto-3-hydroxypropanal on growth of transplantable cancers in mice. Cancer Chemother Rep. 1969 Jun;53(3):195–198. [PubMed] [Google Scholar]
- BERGERET B., BLASCHKO H. The oxidation of cystamine and homocystamine by mammalian enzymes. Br J Pharmacol Chemother. 1957 Dec;12(4):513–516. doi: 10.1111/j.1476-5381.1957.tb00174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BINKLEY F. A note on the specificity of the enzymatic cleavage of thioethers. J Biol Chem. 1951 Sep;192(1):209–211. [PubMed] [Google Scholar]
- Backlund P. S., Jr, Smith R. A. Methionine synthesis from 5'-methylthioadenosine in rat liver. J Biol Chem. 1981 Feb 25;256(4):1533–1535. [PubMed] [Google Scholar]
- Belman S. Onion and garlic oils inhibit tumor promotion. Carcinogenesis. 1983 Aug;4(8):1063–1065. doi: 10.1093/carcin/4.8.1063. [DOI] [PubMed] [Google Scholar]
- Benevenga N. J. Evidence for alternative pathways of methionine catabolism. Adv Nutr Res. 1984;6:1–18. doi: 10.1007/978-1-4613-2801-8_1. [DOI] [PubMed] [Google Scholar]
- Block E. The chemistry of garlic and onions. Sci Am. 1985 Mar;252(3):114–119. doi: 10.1038/scientificamerican0385-114. [DOI] [PubMed] [Google Scholar]
- Blom H. J., van den Elzen J. P., Yap S. H., Tangerman A. Methanethiol and dimethylsulfide formation from 3-methylthiopropionate in human and rat hepatocytes. Biochim Biophys Acta. 1988 Nov 18;972(2):131–136. doi: 10.1016/0167-4889(88)90111-5. [DOI] [PubMed] [Google Scholar]
- Bonomi F., Pagani S., Cerletti P., Cannella C. Rhodanese-Mediated sulfur transfer to succinate dehydrogenase. Eur J Biochem. 1977 Jan 3;72(1):17–24. doi: 10.1111/j.1432-1033.1977.tb11219.x. [DOI] [PubMed] [Google Scholar]
- Bonomi F., Pagani S., Kurtz D. M., Jr Enzymic synthesis of the 4Fe-4S clusters of Clostridium pasteurianum ferredoxin. Eur J Biochem. 1985 Apr 1;148(1):67–73. doi: 10.1111/j.1432-1033.1985.tb08808.x. [DOI] [PubMed] [Google Scholar]
- Branzoli U., Massey V. Evidence for an active site persulfide residue in rabbit liver aldehyde oxidase. J Biol Chem. 1974 Jul 25;249(14):4346–4349. [PubMed] [Google Scholar]
- Broome J. D., Jeng M. W. Promotion of replication in lymphoid cells by specific thiols and disulfides in vitro. Effects on mouse lymphoma cells in comparison with splenic lymphocytes. J Exp Med. 1973 Sep 1;138(3):574–592. doi: 10.1084/jem.138.3.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMMARATA P. S., COHEN P. P. The scope of the transamination reaction in animal tissues. J Biol Chem. 1950 Nov;187(1):439–452. [PubMed] [Google Scholar]
- CAVALLINI D., DE MARCO C., MONDOVI B. Cleavage of cystine by a pyridoxal model. Arch Biochem Biophys. 1960 Apr;87:281–288. doi: 10.1016/0003-9861(60)90173-9. [DOI] [PubMed] [Google Scholar]
- CAVALLINI D., DE MARCO C., MONDOVI B., MORI B. G. The cleavage of cystine by cystathionase and the transulfuration of hypotaurine. Enzymologia. 1960 Sep 1;22:161–173. [PubMed] [Google Scholar]
- CAVALLINI D., DE MARCO C., MONDOVI B. The enzymic conversion of cystamine and thiocysteamine into thiotaurine and hypotaurine. Enzymologia. 1961 May 15;23:101–110. [PubMed] [Google Scholar]
- CAVALLINI D., DE MARCO C., MONDOVI B. The oxidation of cystamine and other sulfur-diamines by diamine-oxidase preparations. Experientia. 1956 Oct 15;12(10):377–379. doi: 10.1007/BF02157276. [DOI] [PubMed] [Google Scholar]
- CAVALLINI D., MONDOVI B., DE MARCO C., SCIOSCIA-SANTORO A. The mechanism of desulphhydration of cysteine. Enzymologia. 1962 Jun 30;24:253–266. [PubMed] [Google Scholar]
- Catignani G. L., Neal R. A. Evidence for the formation of a protein bound hydrodisulfide resulting from the microsomal mixed function oxidase catalyzed desulfuration of carbon disulfide. Biochem Biophys Res Commun. 1975 Jul 22;65(2):629–636. doi: 10.1016/s0006-291x(75)80193-8. [DOI] [PubMed] [Google Scholar]
- Cavallini D., Federici G., Barboni E. Interaction of proteins with sulfide. Eur J Biochem. 1970 May 1;14(1):169–174. doi: 10.1111/j.1432-1033.1970.tb00275.x. [DOI] [PubMed] [Google Scholar]
- Chen S. S., Walgate J. H., Duerre J. A. Oxidative deamination of sulfur amino acids by bacterial and snake venom L-amino acid oxidase. Arch Biochem Biophys. 1971 Sep;146(1):54–63. doi: 10.1016/s0003-9861(71)80040-1. [DOI] [PubMed] [Google Scholar]
- Chen S., Zieve L., Mahadevan V. Mercaptans and dimethyl sulfide in the breath of patients with cirrhosis of the liver. Effect of feeding methionine. J Lab Clin Med. 1970 Apr;75(4):628–635. [PubMed] [Google Scholar]
- Chilcote R. R., Brown E., Rowley J. D. Lymphoblastic leukemia with lymphomatous features associated with abnormalities of the short arm of chromosome 9. N Engl J Med. 1985 Aug 1;313(5):286–291. doi: 10.1056/NEJM198508013130503. [DOI] [PubMed] [Google Scholar]
- Conner J., Russell P. J. Elemental sulfur: a novel inhibitor of adenylate kinase. Biochem Biophys Res Commun. 1983 May 31;113(1):348–352. doi: 10.1016/0006-291x(83)90472-2. [DOI] [PubMed] [Google Scholar]
- DE MARCO C., COLETTA M., CAVALLINI D. Cystine cleavage in alkaline medium. Arch Biochem Biophys. 1963 Jan;100:51–55. doi: 10.1016/0003-9861(63)90033-x. [DOI] [PubMed] [Google Scholar]
- DEMARCO C., BOMBARDIERI G., RIVA F., DUPRE S., CAVALLINI D. (DEGRADATION OF CYSTALDIMINE, THE PRODUCT OF OXIDATIVE DEAMINATION OF CYSTAMINE.) Biochim Biophys Acta. 1965 Apr 12;100:89–97. doi: 10.1016/0304-4165(65)90430-7. [DOI] [PubMed] [Google Scholar]
- Drake M. R., De La Rosa J., Stipanuk M. H. Metabolism of cysteine in rat hepatocytes. Evidence for cysteinesulphinate-independent pathways. Biochem J. 1987 Jun 1;244(2):279–286. doi: 10.1042/bj2440279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein A. L., Kaplan H. S. Feeder layer and nutritional requirements for the establishment and cloning of human malignant lymphoma cell lines. Cancer Res. 1979 May;39(5):1748–1759. [PubMed] [Google Scholar]
- FLAVIN M. Microbial transsulfuration: the mechanism of an enzymatic disulfide elimination reaction. J Biol Chem. 1962 Mar;237:768–777. [PubMed] [Google Scholar]
- Fahey R. C., Mikolajczyk S. D., Meier G. P., Epel D., Carroll E. J., Jr The glutathione thiol-disulfide status in the sea urchin egg during fertilization and the first cell division cycle. Biochim Biophys Acta. 1976 Jul 21;437(2):445–453. doi: 10.1016/0304-4165(76)90013-1. [DOI] [PubMed] [Google Scholar]
- Fitchen J. H., Riscoe M. K., Dana B. W., Lawrence H. J., Ferro A. J. Methylthioadenosine phosphorylase deficiency in human leukemias and solid tumors. Cancer Res. 1986 Oct;46(10):5409–5412. [PubMed] [Google Scholar]
- Fletcher J. C., Robson A. The occurrence of bis-(2-amino-2-carboxyethyl) trisulphide in hydrolysates of wool and other proteins. Biochem J. 1963 Jun;87(3):553–559. doi: 10.1042/bj0870553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furfine E. S., Abeles R. H. Intermediates in the conversion of 5'-S-methylthioadenosine to methionine in Klebsiella pneumoniae. J Biol Chem. 1988 Jul 15;263(20):9598–9606. [PubMed] [Google Scholar]
- GAST J. H., ARAI K., ALDRICH F. L. Quantitative studies on urinary thiosulfate excretion by healthy human subjects. J Biol Chem. 1952 May;196(2):875–884. [PubMed] [Google Scholar]
- GJESSING L. R. STUDIES OF FUNCTIONAL NEURAL TUMORS. II. CYSTATHIONINURIA. Scand J Clin Lab Invest. 1963;15:474–478. [PubMed] [Google Scholar]
- Geiser C. F., Efron M. L. Cystathioninuria in patients with neuroblastoma or ganglioneuroblastoma. Its correlation to vanilmandelic acid excretion and its value in diagnosis and therapy. Cancer. 1968 Oct;22(4):856–860. doi: 10.1002/1097-0142(196810)22:4<856::aid-cncr2820220424>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Geiser C. F., Shih V. E. Cystathioninuria and its origin in children with hepatoblastoma. J Pediatr. 1980 Jan;96(1):72–75. doi: 10.1016/s0022-3476(80)80333-7. [DOI] [PubMed] [Google Scholar]
- Ghoda L. Y., Savarese T. M., Dexter D. L., Parks R. E., Jr, Trackman P. C., Abeles R. H. Characterization of a defect in the pathway for converting 5'-deoxy-5'-methylthioadenosine to methionine in a subline of a cultured heterogeneous human colon carcinoma. J Biol Chem. 1984 Jun 10;259(11):6715–6719. [PubMed] [Google Scholar]
- Glode L. M., Epstein A., Smith C. G. Reduced gamma-cystathionase protein content in human malignant leukemia cell lines as measured by immunoassay with monoclonal antibody. Cancer Res. 1981 Jun;41(6):2249–2254. [PubMed] [Google Scholar]
- Glode L. M., Kriegler M. P., Livingston D. M. Cysteine auxotrophy of human leukemic lymphoblasts is associated with decreased amounts of intracellular cystathionase protein. Biochemistry. 1981 Mar 3;20(5):1306–1311. doi: 10.1021/bi00508a041. [DOI] [PubMed] [Google Scholar]
- Gutteridge S., Tanner S. J., Bray R. C. Comparison of the molybdenum centres of native and desulpho xanthine oxidase. The nature of the cyanide-labile sulphur atom and the nature of the proton-accepting group. Biochem J. 1978 Dec 1;175(3):887–897. doi: 10.1042/bj1750887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HYLIN J. W., WOOD J. L. Enzymatic formation of polysulfides from mercaptopyruvate. J Biol Chem. 1959 Aug;234(8):2141–2144. [PubMed] [Google Scholar]
- Hargrove J. L., Wichman R. D. A cystine-dependent inactivator of tyrosine aminotransferase co-purifies with gamma-cystathionase (cystine desulfurase). J Biol Chem. 1987 May 25;262(15):7351–7357. [PubMed] [Google Scholar]
- Heby O., Gray J. W., Lindl P. A., Marton L. J., Wilson C. B. Changes in L-ornithine decarboxylase activity during the cell cycle. Biochem Biophys Res Commun. 1976 Jul 12;71(1):99–105. doi: 10.1016/0006-291x(76)90254-0. [DOI] [PubMed] [Google Scholar]
- Helson L., Fleisher M., Bethune V., Murphy M. L., Schwartz M. K. Urinary cystathionine, catecholamine, and metabolites in patients with neuroblastoma. Clin Chem. 1972 Jul;18(7):613–615. [PubMed] [Google Scholar]
- Hoffman R. M., Erbe R. W. High in vivo rates of methionine biosynthesis in transformed human and malignant rat cells auxotrophic for methionine. Proc Natl Acad Sci U S A. 1976 May;73(5):1523–1527. doi: 10.1073/pnas.73.5.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman R. M. Methionine dependence in cancer cells - a review. In Vitro. 1982 May;18(5):421–428. doi: 10.1007/BF02796468. [DOI] [PubMed] [Google Scholar]
- JACKSON J. F., LINDAHL-KIESSLING K. ACTION OF SULFHYDRYL COMPOUNDS ON HUMAN LEUKOCYTE MITOSIS IN VITRO. Exp Cell Res. 1964 May;34:515–524. doi: 10.1016/0014-4827(64)90237-x. [DOI] [PubMed] [Google Scholar]
- Jackson J. F., Hill F. S. Polyploidy in human leucocyte cultures treated with cysteamine and irradiation. Nature. 1967 Jun 10;214(5093):1155–1156. doi: 10.1038/2141155a0. [DOI] [PubMed] [Google Scholar]
- KEARNEY E. B., SINGER T. P. Enzymic transformations of L-cysteinesulfinic acid. Biochim Biophys Acta. 1953 Jun;11(2):276–289. doi: 10.1016/0006-3002(53)90037-7. [DOI] [PubMed] [Google Scholar]
- Kadota H., Ishida Y. Production of volatile sulfur compounds by microorganisms. Annu Rev Microbiol. 1972;26:127–138. doi: 10.1146/annurev.mi.26.100172.001015. [DOI] [PubMed] [Google Scholar]
- Kamatani N., Yu A. L., Carson D. A. Deficiency of methylthioadenosine phosphorylase in human leukemic cells in vivo. Blood. 1982 Dec;60(6):1387–1391. [PubMed] [Google Scholar]
- Kamely D., Weissbach H., Kerwar S. S. Methionine biosynthesis in normal and transformed fibroblasts. Arch Biochem Biophys. 1977 Feb;179(1):43–45. doi: 10.1016/0003-9861(77)90084-4. [DOI] [PubMed] [Google Scholar]
- Kano Y., Sakamoto S., Kasahara T., Kusumoto K., Hida K., Suda K., Ozawa K., Miura Y., Takaku F. Methionine dependency of cell growth in normal and malignant hematopoietic cells. Cancer Res. 1982 Aug;42(8):3090–3092. [PubMed] [Google Scholar]
- Kato A., Ogura M., Suda M. Control mechanism in the rat liver enzyme system converting L-methionine to L-cystine. 3. Noncompetitive inhibition of cystathionine synthetase-serine dehydratase by elemental sulfur and competitive inhibition of cystathionase-homoserine dehydratase by L-cysteine and L-cystine. J Biochem. 1966 Jan;59(1):40–48. doi: 10.1093/oxfordjournals.jbchem.a128256. [DOI] [PubMed] [Google Scholar]
- Klein C. E., Roberts B., Holcenberg J., Glode L. M. Cystathionine metabolism in neuroblastoma. Cancer. 1988 Jul 15;62(2):291–298. doi: 10.1002/1097-0142(19880715)62:2<291::aid-cncr2820620211>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Kreis W., Goodenow M. Methionine requirement and replacement by homocysteine in tissue cultures of selected rodent and human malignant and normal cells. Cancer Res. 1978 Aug;38(8):2259–2262. [PubMed] [Google Scholar]
- Kuchino Y., Yasuda T., Nishimura S. Deficiency of S-adenosylmethionine-homocysteine methyltransferase activity in hepatoma cells. Cancer Res. 1977 Jan;37(1):206–208. [PubMed] [Google Scholar]
- Kusunoki S., Yasumasu I. Cyclic change in polyamine concentrations in sea urchin eggs related with cleavage cycle. Biochem Biophys Res Commun. 1976 Feb 9;68(3):881–885. doi: 10.1016/0006-291x(76)91227-4. [DOI] [PubMed] [Google Scholar]
- Lang J. M., Touraine J. L., Trepo C., Choutet P., Kirstetter M., Falkenrodt A., Herviou L., Livrozet J. M., Retornaz G., Touraine F. Randomised, double-blind, placebo-controlled trial of ditiocarb sodium ('Imuthiol') in human immunodeficiency virus infection. Lancet. 1988 Sep 24;2(8613):702–706. doi: 10.1016/s0140-6736(88)90184-5. [DOI] [PubMed] [Google Scholar]
- Lau B. H., Woolley J. L., Marsh C. L., Barker G. R., Koobs D. H., Torrey R. R. Superiority of intralesional immunotherapy with Corynebacterium parvum and Allium sativum in control of murine transitional cell carcinoma. J Urol. 1986 Sep;136(3):701–705. doi: 10.1016/s0022-5347(17)45031-2. [DOI] [PubMed] [Google Scholar]
- Lipsett M. N. Biosynthesis of 4-thiouridylate. Participation of a sulfurtransferase containing pyridoxal 5'-phosphate. J Biol Chem. 1972 Mar 10;247(5):1458–1461. [PubMed] [Google Scholar]
- Lipsett M. N., Norton J. S., Peterkofsky A. A requirement for beta-mercaptopyruvate in the in vitro thiolation of transfer ribonucleic acid. Biochemistry. 1967 Mar;6(3):855–860. doi: 10.1021/bi00855a028. [DOI] [PubMed] [Google Scholar]
- Livingston D. M., Ferguson C., Gollogly R., Lazarus H. Accumulation of cystine auxotrophic thymocytes accompanying type C viral leukemogenesis in the mouse. Cell. 1976 Jan;7(1):41–47. doi: 10.1016/0092-8674(76)90253-1. [DOI] [PubMed] [Google Scholar]
- Marchitto K. S., Ferro A. J. The metabolism of 5'-methylthioadenosine and 5-methylthioribose 1-phosphate in Saccharomyces cerevisiae. J Gen Microbiol. 1985 Sep;131(9):2153–2164. doi: 10.1099/00221287-131-9-2153. [DOI] [PubMed] [Google Scholar]
- Marquardt H., Sapozink M. D., Zedeck M. S. Inhibition by cysteamine-HCl of oncogenesis induced by 7,12-dimethylbenz(alpha)anthracene without affecting toxicity. Cancer Res. 1974 Dec;34(12):3387–3390. [PubMed] [Google Scholar]
- Massey V., Edmondson D. On the mechanism of inactivation of xanthine oxidase by cyanide. J Biol Chem. 1970 Dec 25;245(24):6595–6598. [PubMed] [Google Scholar]
- Massey V., Williams C. H., Jr, Palmer G. The presence of S degrees-containing impurities in commercial samples of oxidized glutathione and their catalytic effect on the reduction of cytochrome c. Biochem Biophys Res Commun. 1971 Feb 19;42(4):730–738. doi: 10.1016/0006-291x(71)90548-1. [DOI] [PubMed] [Google Scholar]
- Masuda Y., Yasoshima M., Shibata K. Effects of carbon disulfide, diethyldithiocarbamate, and disulfiram on drug metabolism in the perfused rat liver. Res Commun Chem Pathol Pharmacol. 1988 Jul;61(1):65–82. [PubMed] [Google Scholar]
- Matsumoto K. E., Partridge D. H., Robinson A. B., Pauling L., Flath R. A., Mon T. R., Teranishi R. The identification of volatile compounds in human urine. J Chromatogr. 1973 Oct 10;85(1):31–34. doi: 10.1016/s0021-9673(01)91861-8. [DOI] [PubMed] [Google Scholar]
- McCann P. P., Tardiff C., Mamont P. S., Schuber F. Biphasic induction of ornithine decarboxylase and putrescine levels in growing HTC cells. Biochem Biophys Res Commun. 1975 May 5;64(1):336–341. doi: 10.1016/0006-291x(75)90258-2. [DOI] [PubMed] [Google Scholar]
- Mills G. C., Mills J. S. Urinary excretion of methylthioadenosine in immunodeficient children. Clin Chim Acta. 1985 Mar 30;147(1):15–23. doi: 10.1016/0009-8981(85)90005-1. [DOI] [PubMed] [Google Scholar]
- Mohindru A., Fisher J. M., Rabinovitz M. Endogenous copper is cytotoxic to a lymphoma in primary culture which requires thiols for growth. Experientia. 1985 Aug 15;41(8):1064–1066. doi: 10.1007/BF01952146. [DOI] [PubMed] [Google Scholar]
- Murakami Y., Kameji T., Hayashi S. Cysteine-dependent inactivation of hepatic ornithine decarboxylase. Biochem J. 1984 Jan 15;217(2):573–580. doi: 10.1042/bj2170573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niukian K., Schwartz J., Shklar G. Effects of onion extract on the development of hamster buccal pouch carcinomas as expressed in tumor burden. Nutr Cancer. 1987;9(2-3):171–176. doi: 10.1080/01635588709513924. [DOI] [PubMed] [Google Scholar]
- Pagani S., Bonomi F., Cerletti P. Enzymic synthesis of the iron-sulfur cluster of spinach ferredoxin. Eur J Biochem. 1984 Jul 16;142(2):361–366. doi: 10.1111/j.1432-1033.1984.tb08295.x. [DOI] [PubMed] [Google Scholar]
- Pagani S., Eldridge M., Eady R. R. Nitrogenase of Klebsiella pneumoniae. Rhodanese-catalysed restoration of activity of the inactive 2Fe species of the Fe protein. Biochem J. 1987 Jun 1;244(2):485–488. doi: 10.1042/bj2440485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagani S., Galante Y. M. Interaction of rhodanese with mitochondrial NADH dehydrogenase. Biochim Biophys Acta. 1983 Jan 26;742(2):278–284. doi: 10.1016/0167-4838(83)90312-6. [DOI] [PubMed] [Google Scholar]
- Pestaña A., Sols A. Reversible inactivation by elemental sulfur and mercurials of rat liver serine dehydratase and certain sulfhydryl enzymes. Biochem Biophys Res Commun. 1970 May 11;39(3):522–529. doi: 10.1016/0006-291x(70)90609-1. [DOI] [PubMed] [Google Scholar]
- Petering D., Fee J. A., Palmer G. The oxygen sensitivity of spinach ferredoxin and other iron-sulfur proteins. The formation of protein-bound sulfur-zero. J Biol Chem. 1971 Feb 10;246(3):643–653. [PubMed] [Google Scholar]
- Ploegman J. H., Drent G., Kalk K. H., Hol W. G., Heinrikson R. L., Keim P., Weng L., Russell J. The covalent and tertiary structure of bovine liver rhodanese. Nature. 1978 May 11;273(5658):124–129. doi: 10.1038/273124a0. [DOI] [PubMed] [Google Scholar]
- Pompidou A., Delsaux M. C., Telvi L., Mace B., Coutance F., Falkenrodt A., Lang J. M. Isoprinosine and Imuthiol, two potentially active compounds in patients with AIDS-related complex symptoms. Cancer Res. 1985 Sep;45(9 Suppl):4671s–4673s. [PubMed] [Google Scholar]
- Poste G. Mechanisms of virus-induced cell fusion. Int Rev Cytol. 1972;33:157–252. doi: 10.1016/s0074-7696(08)61451-5. [DOI] [PubMed] [Google Scholar]
- Rajnherc J. R., van Gennip A. H., Abeling N. G., van der Zee J. M., Voûte P. A. Cystathioninuria in patients with neuroblastoma. Med Pediatr Oncol. 1984;12(2):81–84. doi: 10.1002/mpo.2950120203. [DOI] [PubMed] [Google Scholar]
- Renoux G., Renoux M. Thymus-like activities of sulphur derivatives on T-cell differentiation. J Exp Med. 1977 Feb 1;145(2):466–471. doi: 10.1084/jem.145.2.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci G., Nardini M., Federici G., Cavallini D. The transamination of L-cystathionine, L-cystine and related compounds by a bovine kidney transaminase. Eur J Biochem. 1986 May 15;157(1):57–63. doi: 10.1111/j.1432-1033.1986.tb09637.x. [DOI] [PubMed] [Google Scholar]
- Russell P. J., Conner J., Sisson S. Sulfur specifically inhibits adenylate kinase in assays for creatine kinase. Clin Chem. 1984 Sep;30(9):1555–1557. [PubMed] [Google Scholar]
- SAKAI H., DAN K. Studies on sulfhydryl groups during cell division of sea urchin egg. I. Glutatione. Exp Cell Res. 1959 Jan;16(1):24–41. doi: 10.1016/0014-4827(59)90192-2. [DOI] [PubMed] [Google Scholar]
- SORBO B. A colorimetric method for the determination of thiosulfate. Biochim Biophys Acta. 1957 Feb;23(2):412–416. doi: 10.1016/0006-3002(57)90346-3. [DOI] [PubMed] [Google Scholar]
- SORBO B. Enzymic transfer of sulfur from mercaptopyruvate to sulfate or sulfinates. Biochim Biophys Acta. 1957 May;24(2):324–329. doi: 10.1016/0006-3002(57)90201-9. [DOI] [PubMed] [Google Scholar]
- STERN H. Variations in sulfhydryl concentration during microsporocyte meiosis in the anthers of Lilium and Trillium. J Biophys Biochem Cytol. 1958 Mar 25;4(2):157–161. doi: 10.1083/jcb.4.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandy J. D., Davies R. C., Neuberger A. Control of 5-aminolaevulinate synthetase activity in Rhodopseudomonas spheroides a role for trisulphides. Biochem J. 1975 Aug;150(2):245–257. doi: 10.1042/bj1500245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawahata T., Neal R. A. Inhibition of rat liver cytochrome P-450 by benzyl hydrodisulfide. Mol Pharmacol. 1982 Mar;21(2):464–467. [PubMed] [Google Scholar]
- Silver M., Lundgren D. G. The thiosulfate-oxidizing enzyme of Ferrobacillus ferrooxidans (Thiobacillus ferrooxidans). Can J Biochem. 1968 Oct;46(10):1215–1220. doi: 10.1139/o68-181. [DOI] [PubMed] [Google Scholar]
- Sparnins V. L., Barany G., Wattenberg L. W. Effects of organosulfur compounds from garlic and onions on benzo[a]pyrene-induced neoplasia and glutathione S-transferase activity in the mouse. Carcinogenesis. 1988 Jan;9(1):131–134. doi: 10.1093/carcin/9.1.131. [DOI] [PubMed] [Google Scholar]
- Steele R. D., Benevenga N. J. Identification of 3-methylthiopropionic acid as an intermediate in mammalian methionine metabolism in vitro. J Biol Chem. 1978 Nov 10;253(21):7844–7850. [PubMed] [Google Scholar]
- Steele R. D., Benevenga N. J. The metabolism of 3-methylthiopropionate in rat liver homogenates. J Biol Chem. 1979 Sep 25;254(18):8885–8890. [PubMed] [Google Scholar]
- Sunkara P. S., Chang C. C., Lachman P. J. Cell proliferation and cell cycle dependent changes in the methylthioadenosine phosphorylase activity in mammalian cells. Biochem Biophys Res Commun. 1985 Mar 15;127(2):546–551. doi: 10.1016/s0006-291x(85)80194-7. [DOI] [PubMed] [Google Scholar]
- Suzuki I. Oxidation of elemental sulfur by an enzyme system of Thiobacillus thiooxidans. Biochim Biophys Acta. 1965 Jul 8;104(2):359–371. doi: 10.1016/0304-4165(65)90341-7. [DOI] [PubMed] [Google Scholar]
- Szczepkowski T. W., Wood J. L. The cystathionase-rhodanese system. Biochim Biophys Acta. 1967 Jul 11;139(2):469–478. doi: 10.1016/0005-2744(67)90050-2. [DOI] [PubMed] [Google Scholar]
- Taniguchi T., Kimura T. Role of 3-mercaptopyruvate sulfurtransferase in the formation of the iron-sulfur chromophore of adrenal ferredoxin. Biochim Biophys Acta. 1974 Oct 17;364(2):284–295. doi: 10.1016/0005-2744(74)90014-x. [DOI] [PubMed] [Google Scholar]
- Tatsuta M., Iishi H., Yamamura H., Baba M., Mikuni T., Taniguchi H. Inhibitory effect of prolonged administration of cysteamine on experimental carcinogenesis in rat stomach induced by N-methyl-N'-nitro-N-nitrosoguanidine. Int J Cancer. 1988 Mar 15;41(3):423–426. doi: 10.1002/ijc.2910410318. [DOI] [PubMed] [Google Scholar]
- Toohey J. I., Cline M. J. Alkylthiolation. Evidence for involvement in cell division. Biochem Biophys Res Commun. 1976 Jun 21;70(4):1275–1282. doi: 10.1016/0006-291x(76)91040-8. [DOI] [PubMed] [Google Scholar]
- Toohey J. I. Ketomethylthiobutyric acid formation from methylthioadenosine: a diffusion assay. Arch Biochem Biophys. 1983 Jun;223(2):533–542. doi: 10.1016/0003-9861(83)90618-5. [DOI] [PubMed] [Google Scholar]
- Toohey J. I. Macrophages and methylthio groups in lymphocyte proliferation. J Supramol Struct Cell Biochem. 1981;17(1):11–25. doi: 10.1002/jsscb.380170103. [DOI] [PubMed] [Google Scholar]
- Toohey J. I. Methylthioadenosine nucleoside phosphorylase deficiency in methylthio-dependent cancer cells. Biochem Biophys Res Commun. 1978 Jul 14;83(1):27–35. doi: 10.1016/0006-291x(78)90393-5. [DOI] [PubMed] [Google Scholar]
- Toohey J. I. Persulfide sulfur is a growth factor for cells defective in sulfur metabolism. Biochem Cell Biol. 1986 Aug;64(8):758–765. doi: 10.1139/o86-103. [DOI] [PubMed] [Google Scholar]
- Toohey J. I. Sulfhydryl dependence in primary explant hematopoietic cells. Inhibition of growth in vitro with vitamin B12 compounds. Proc Natl Acad Sci U S A. 1975 Jan;72(1):73–77. doi: 10.1073/pnas.72.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubuka T., Ishimoto Y., Akagi R. Transaminative metabolism of L-cysteine in rat tissues. J Inherit Metab Dis. 1981;4(2):65–66. doi: 10.1007/BF02263593. [DOI] [PubMed] [Google Scholar]
- Ubuka T., Ishimoto Y., Kasahara K. Determination of 3-mercaptopyruvate-cysteine disulfide, a product of oxidative deamination of L-cystine by L-amino acid oxidase. Anal Biochem. 1975 Jul;67(1):66–73. doi: 10.1016/0003-2697(75)90272-9. [DOI] [PubMed] [Google Scholar]
- VILLAREJO M., WESTLEY J. MECHANISM OF RHODANESE CATALYSIS OF THIOSULFATE-LIPOATE OXIDATION-REDUCTION. J Biol Chem. 1963 Dec;238:4016–4020. [PubMed] [Google Scholar]
- Valentine W. N., Toohey J. I., Paglia D. E., Nakatani M., Brockway R. A. Modification of erythrocyte enzyme activities by persulfides and methanethiol: possible regulatory role. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1394–1398. doi: 10.1073/pnas.84.5.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vennesland B., Castric P. A., Conn E. E., Solomonson L. P., Volini M., Westley J. Cyanide metabolism. Fed Proc. 1982 Aug;41(10):2639–2648. [PubMed] [Google Scholar]
- Voûte P. A., Jr, Wadman S. K. Cystathioninuria in hepatoblastoma. Clin Chim Acta. 1968 Nov;22(3):373–378. doi: 10.1016/0009-8981(68)90038-7. [DOI] [PubMed] [Google Scholar]
- WEISBERGER A. S., PENSKY J. Tumor inhibition by a sulfhydryl-blocking agent related to an active principle of garlic (Allium sativum). Cancer Res. 1958 Dec;18(11):1301–1308. [PubMed] [Google Scholar]
- WILLS E. D. Enzyme inhibition by allicin, the active principle of garlic. Biochem J. 1956 Jul;63(3):514–520. doi: 10.1042/bj0630514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl R. C., Rajagopalan K. V. Evidence for the inorganic nature of the cyanolyzable sulfur of molybdenum hydroxylases. J Biol Chem. 1982 Feb 10;257(3):1354–1359. [PubMed] [Google Scholar]
- Wargovich M. J. Diallyl sulfide, a flavor component of garlic (Allium sativum), inhibits dimethylhydrazine-induced colon cancer. Carcinogenesis. 1987 Mar;8(3):487–489. doi: 10.1093/carcin/8.3.487. [DOI] [PubMed] [Google Scholar]
- Wong T. W., Harris M. A., Jankowicz C. A. Transfer ribonucleic acid sulfurtransferase isolated from rat cerebral hemispheres. Biochemistry. 1974 Jul 2;13(14):2805–2812. doi: 10.1021/bi00711a004. [DOI] [PubMed] [Google Scholar]
- Wong T. W., Harris M. A., Morris H. P. The presence of an inhibitor of RNA sulfurtransferase in Morris hepatomas. Biochem Biophys Res Commun. 1975 Aug 4;65(3):1137–1145. doi: 10.1016/s0006-291x(75)80504-3. [DOI] [PubMed] [Google Scholar]
- Wong T. W., Weiss S. B., Eliceiri G. L., Bryant J. Ribonucleic acid sulfurtransferase from Bacillus subtilis W168. Sulfuration with beta-mercaptopyruvate and properties of the enzyme system. Biochemistry. 1970 May 26;9(11):2376–2386. doi: 10.1021/bi00813a024. [DOI] [PubMed] [Google Scholar]
- Wood J. L. Biochemical functions of persulfides. Adv Exp Med Biol. 1982;148:327–342. doi: 10.1007/978-1-4615-9281-5_26. [DOI] [PubMed] [Google Scholar]
- Wood J. L. Sulfane sulfur. Methods Enzymol. 1987;143:25–29. doi: 10.1016/0076-6879(87)43009-7. [DOI] [PubMed] [Google Scholar]
- Yamanishi T., Kubota I., Tuboi S. Mechanism of the activation of delta-aminolevulinate synthetase in Rhodopseudomonas spheroides by rat liver mitochondrial fraction. J Biochem. 1983 Jul;94(1):181–188. doi: 10.1093/oxfordjournals.jbchem.a134328. [DOI] [PubMed] [Google Scholar]
- Yamanishi T., Tuboi S. The mechanism of the L-cystine cleavage reaction catalyzed by rat liver gamma-cystathionase. J Biochem. 1981 Jun;89(6):1913–1921. doi: 10.1093/oxfordjournals.jbchem.a133393. [DOI] [PubMed] [Google Scholar]
- You W. C., Blot W. J., Chang Y. S., Ershow A., Yang Z. T., An Q., Henderson B. E., Fraumeni J. F., Jr, Wang T. G. Allium vegetables and reduced risk of stomach cancer. J Natl Cancer Inst. 1989 Jan 18;81(2):162–164. doi: 10.1093/jnci/81.2.162. [DOI] [PubMed] [Google Scholar]
- Zlatkis A., Liebich H. M. Profile of volatile metabolites in human urine. Clin Chem. 1971 Jul;17(7):592–594. [PubMed] [Google Scholar]
- von Studnitz W. Cystathioninuria in children with neuroblastoma with and without metastasis. Acta Paediatr Scand. 1970 Jan;59(1):80–82. doi: 10.1111/j.1651-2227.1970.tb15518.x. [DOI] [PubMed] [Google Scholar]