Abstract
Background
Understanding the shelf life of wine is complex and involves factors such as aroma preservation, flavour development and market acceptance. Ageing potential, crucial for flavour complexity, exposes wine to oxidation, influenced by oxygen, temperature and light, with an impact on quality. This type of oxidation is non-enzymatic, is catalyzed by metal ions and alters colour and flavour.
Scope and approach
This review examines the dynamics of wine preservation, focusing on oxidation and the impact of closure. Corks allow controlled oxygen transfer, while screw caps offer a nearly hermetic closure. Oxygen transfer rates vary, with natural corks having fluctuating rates and synthetic corks causing over-exposure. Additives such as sulphur dioxide and alternative substitute such as lysozyme and ascorbic acid are examined for their role in preventing oxidation and ensuring microbiological stability.
Key findings and conclusions
Closure choice significantly affects wine preservation. Balancing oxygen exposure, temperature, and light is vital. Effective management, including the strategic use of preservatives and additives, is crucial for maintaining quality and extending shelf life. This review underscores the delicate equilibrium necessary for preserving wine quality from production to consumption.
Keywords: Oxygen, Sulphur dioxide, Alternative additives, Capping
Highlights
-
•
The article delves into the multifaceted aspects of determining and extending the wine shelf life, considering factors like flavour retention, character progression, and commercial viability.
-
•
Oxygen dual role in wine production, both beneficial and detrimental, is explored. While it aids in yeast fermentation and polymerization of polyphenolic components, excessive exposure can lead to oxidation, negatively affecting wine features.
-
•
SO2 serves as antioxidant and antimicrobial agent, while alternative additives aim to enhance antioxidant capacity and mitigate oxidation risks.
1. Introduction
The shelf life (SL) of a food product typically refers to the duration within which it remains safe for consumption, respecting the nutritional information on the label, while retaining its desired sensory, chemical, physical, and microbiological attributes when stored under recommended conditions [1,2]. The shelf life endpoint is influenced by various factors, including legal requirements such as the 80/80 rule for nutritional loss in natural foods, consumer taste preferences, distribution demands, and cost considerations. From the food industry perspective, shelf life is measured by the level of acceptable deterioration of the quality before consumption, which often remains organoleptically satisfactory. However, for consumers, the shelf life ends when the food no longer maintains an acceptable taste [3,4] and thus extending the SL is crucial for both consumers and producers [[5], [6], [7], [8], [9]]. Defining the shelf life of wine, therefore, represents a difficult challenge compared to other products.
The quality and the shelf life of wine are strongly influenced by the oxidative reaction on wine polyphenols.
Phenolic compounds are at the utmost importance in wine as they contribute to different organolectic characters, like colour, aroma, astringency, and bitterness [10]. Wine polyphenols are classified into flavonoids and non-flavonoids. Flavonoids, such as flavanols (e.g., quercetin), flavan-3-ols (e.g., catechin), and anthocyanins (e.g., malvidin-3-glucoside), contribute to wine physicochemical properties and stability [11], and their concentration is influenced by winemaking practices. Those bioactive compounds play a key role in oxidative processes. Indeed, wine oxidation may reduce their content, thus reducing the beneficial effects on human health to which they are related [12]. The oxygenation of red wine causes the loss of monomeric anthocyanins and the formation of SO2-resistant polymeric pigments [13], comprising components that contribute to colour, taste, and mouthfeel [14]. Additionally, oxygen presence may catalyze phenolic breakdown, leading to a loss of antioxidant capacity and off-flavours [15]. The rate of oxidation and its impact on wine quality are influenced by factors like oxygen exposure, storage temperature and light, and metal ions. Winemakers employ strategies like controlled oxygen exposure, antioxidants like sulphur dioxide, and optimal storage conditions, to manage oxidation and preserve wine quality over time [16].
For shelf life, the maintenance of the initial flavour or their evolution un the appropriate manner is also detrimental [[17], [18], [19]]. For wines designed for immediate consumption, the maintenance of unchanged flavour characters is expected. Initially, post-bottling flavour alterations may be advantageous, with the disappearance of residual yeasty odours and a decreased bitterness and astringency [19]. However, subsequent changes often lead to the gradual fading of fresh fruity or floral aspects, eventually replaced by an aged bouquet in wines with aging potential. If off-odours are not developed while the initial flavours are replaced by aged bouquets, the shelf life may range from a few months to years, rather than decades [20].
Another important compound for understand wine oxidation is acetaldehyde (CH3CHO) is a key volatile compound in wine [[21], [22], [23], [24]] constituting approximately 90 % of the total aldehyde content in wine [25,26] and its formation, beside being related to the yeast fermentation activity, is directly linked to wine oxidation levels and oxygen exposure. Red wines typically contain around 30 mg/L, white wines 80 mg/L, and Sherries 300 mg/L [27], with Sherries distinguished by their high acetaldehyde levels [28,29]. At lower concentrations, acetaldehyde provides pleasant fruity aromas, but at higher levels it is responsible for a scent reminiscent of rotten apples [30]. The acetaldehyde threshold in wine varies between 100 and 125 mg/L.
The loss of these desirable characteristics in wine is often attributed to poor storage conditions, including exposure to oxygen [20,26,[31], [32], [33]], unsuitable temperatures [25,34], or prolonged light exposure [25,[35], [36], [37]]. Several studies reported different volatile compounds, including acetaldehyde, as components linked to oxidized characters and therefore analytical techniques based on metabolomic approach, like HS-SPME/GC-MS, represent important tools to detect poor quality wine [38,39].
1.1. Effect of oxygen
The presence of oxygen in wine can be considered either positive or negative depending on the moment of the production process and the effect it has on the product [40]. Oxygen determines positive effects as it enhances the yeast multiplication during the fermentation of the must, improves the polymerization of the polyphenolic component, and eliminates the reduced odours [41,42]. Conversely, it is negative for the oxidation of aromatic compounds [43], ethanol, phenols, and especially anthocyanins [11].
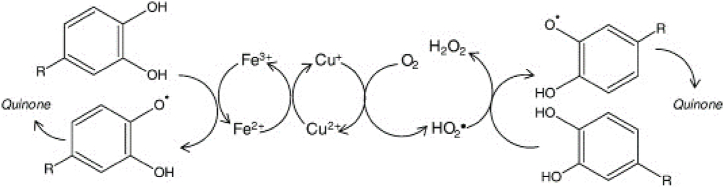
Oxidation processes in wines can proceed through enzymatic and non-enzymatic pathways [44]. Enzymatic oxidation occurs mainly in the wine must during pressing and alcoholic fermentation and it is facilitated by various oxidoreductase enzymes, such as laccase, catechol oxidase, and monophenol monooxygenase [11,45,46]. A probable oxidation mechanism of phenolic compounds provides the hydroxylation at the ortho position of an existing hydroxyl group of the phenolic molecule and the oxidation of ortho-dihydroxybenzenes to ortho-benzoquinones (Fig. 1) [11].
Fig. 1.
Enzymatic oxidation process [47].
Consequently, the oxidation mechanism that leads to changes during bottled storage is predominantly non-enzymatic and involves the breakdown of wine components by oxygen [46]. It is widely recognized that this oxidative chain reaction is catalyzed by metal ions [48], particularly iron (Fe), copper (Cu) and manganese (Mn), with iron playing a central role [49]. The concentrations of these inorganic elements in wine are influenced by different factors, such as grape variety, growing conditions, and winemaking techniques, including grape pressing, must cutting, and filtration methods [50].
Oxidative phenomena may therefore explain the colour changes [16,20,42,51] undergone by wine during maturation. Indeed, free anthocyanins, responsible for purplish highlights, are very high in young red wines and show a tendency to decrease significantly during refinement due to oxidative phenomena, copolymerization with other phenols, and polymerization between non- and oxidized forms [52], determining colour changes towards garnet red [53] (Fig. 2). During the non-enzymatic oxidation process of wine, o-diphenols are oxidized to o-quinones and semi-quinone, and free radicals may be produced, while oxygen is reduced to H2O2. Quinones formed during the process as primary products are unstable and may undergo further reactions. These reactions, which can lead to pigment formation, are similar to those occurring in enzymatic browning reactions.
Fig. 2.
Catalytic involvement of iron and copper ions in the phenolic oxidation to produce quinones and hydrogen peroxide [54].
The successful development of a wine ageing process is thus related to both the diffusive process that takes place with the extraction of noble compounds from the wood [[55], [56], [57]] and the initial composition of the wine that has been introduced into the container. A wine capable of sustaining ageing in wood should be transferred to the barrel as soon as possible immediately after malolactic fermentation, and its evolution will be the faster the lower capacity of the barrel (greater content of dissolved oxygen per unit volume of liquid) [58]. Since the laws regulating the barrel ageing process are not yet sufficiently known, the proper evolution of the product remains a random character, even though, in principle, it may be asserted that a wine with a high content of polyphenols, especially tannins [59], may respond better than one poorer in these components, which instead risks to bee too loaded of fruity aromas from wood and with advanced oxidative characters [60].
However, although wood is not essential for the ageing of every type of wine [61], it usually determines an improvement in bouquet and roundness on the palate, which makes the wines broader and more elegant. This is due to the fact that reduced substances, the nature of which has not yet been fully elucidated, are formed in the bottle and are essential for the development of the mentioned characteristics [62]. These substances, showing pleasant aroma features, are very sensitive to oxygen, and this is why mature wines show considerable evolutions in the glass in few hours after being uncorked [63], before become flat and vanished.
Thus, a phase of wine maturation in containers where a reducing environment can be achieved result essential for the development of characteristics perceived as pleasant by consumers, and therefore the research for appropriate corking systems, able to create proper conditions without transferring undesirable characters to the wine, is at the utmost importance [3,64,65]. Indeed, some authorities highlight that the cork could be considered as one of the variables that serve to influence the style of wine to be produced [19].
It is well known that the effect of oxygen on the entire wine production process is a topic of enormous interest to all the subjects operating in the wine industry, as demonstrated by the abundant literature on the subject.
Oxygen, present as a gaseous element at standard temperature and pressure, constitutes approximately 21 % of the Earth's atmosphere [66]. Its most stable form, known as triplet oxygen (although correctly termed dioxygen), features two split electrons in different molecular orbitals of the diradical molecule with parallel spins. In its triplet state, oxygen is relatively inert and stable compared to most radicals [67].
The conventional Lewis structure depicting oxygen as a double bond (O=O) does not accurately represent its diradical nature; a more precise representation is a simple bond (-O-O-) [68], where the two split electrons are shared. In this electronic arrangement, oxygen cannot readily react with the majority of organic molecules or form bonds by accepting electron pairs. However, it is not entirely inert and can participate in certain organic and inorganic reactions, such as reacting with other radicals to form new radicals. Conversion of oxygen to its excited singlet state, either chemically, thermally, or through light exposure, weakens the O–O bond and removes spin restriction [69,70].
After absorbing sufficient energy, oxygen transits to the singlet state (designated 1O2), where its electron pair possesses antiparallel spin. Although not a free radical, singlet oxygen species is highly reactive [8] and electrophilic, able to readily form chemical bonds with other molecules leading to oxidative reactions. The reaction rate of singlet oxygen with organic molecules is significantly higher than that of triplet oxygen due to its lower activation energy [71].
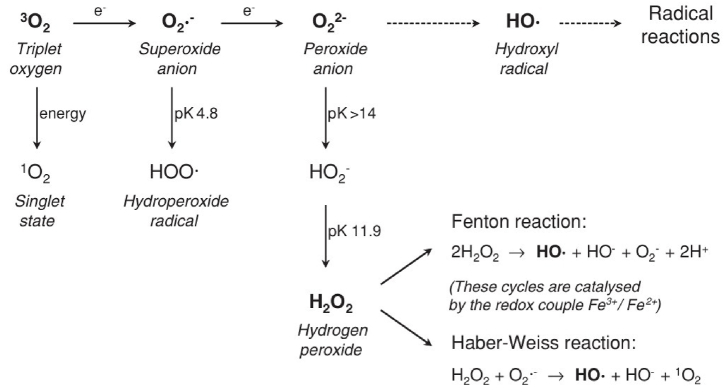
The reactivity of a molecule correlates with its half-life in a given medium, with 1O2 having a half-life of approximately 1 μs in water [70,[72], [73], [74]]. In an aqueous environment, the monovalent reduction of triplet oxygen by accepting a single electron can lead to the formation of a superoxide anion (O2·−), typically catalyzed by metal ions like Fe2+/Fe3+. While this activation is not probable in wine conditions without light, it is generally associated with iron catalysis [75].
The resulting radical can act as both an oxidant and a reductant agent. Indeed oxygen exists as two different radicals, one existing as protonated form of the hydroperoxide radical (HOO-) at wine pH [76] that is less reactive, but serves as a precursor of the most reactive oxygen species that is the hydroxyl radical (HO·) [77]. While the initial stage of oxygen reduction is endothermic and rate-limiting, subsequent stages are exothermic and spontaneous [32]. In the presence of metal ions like Fe2+/Fe3+ or Cu+/Cu2+, the superoxide anion can further undergo reduction to produce peroxide anion (O22−) [76], which readily protonates into hydrogen peroxide (H2O2) at wine pH [76,78].
Additionally, in the presence of metal ions, the Fenton reaction or the Haber-Weiss reaction (Fig. 3) [32,79] can generate a hydroxyl radical and a hydroxide ion from H2O2 [80], yielding highly reactive oxygen species [81] from a weakly reactive precursor [32,62].
Fig. 3.
Formation of reactive species [32].
According to the above mentioned mechanism, polyphenols represent one of the main substrate of oxidation reactions in wine [11], both during the production process and during ageing in addition with sulphur dioxide (SO2) [54]. Oxidative reactions on polyphenols can lead to defects in the organoleptic characteristics of wines, such as changes in colour, aroma, and increase of the sensation of astringency [75].
1.2. Effect of temperature
Temperature plays a crucial role in wine storage as it influences the pace of chemical reactions. This relationship is elucidated by the Arrhenius equation (Eq. (1)) [82]:
| (1) |
Where:
k: kinetic constant;
A: pre-emption factor, which remains constant under moderate temperature fluctuations, determined by the collision frequency and steric factor, also referred to as frequency factor;
Ea: activation energy, another constant at moderate temperatures;
R: gas constant;
T: absolute temperature.
According to the Arrhenius equation, increased temperatures lead to a corresponding increase in the rate of reaction within the product [83,84].
A study by Oliveira et al. [85], showed maximum degradation of phenolic compounds (anthocyanins) was achieved at higher temperatures and followed first-order kinetics (Fig. 4(A–C). In the context of wine, temperature is crucial not only during fermentation but also during storage [34].
Fig. 4.
Arrhenius plot for delphinidin-3-glucoside (Dp-3G) and petunidin-3-glucoside (Pt-3G) depletion (A); for peonidin-3-glucoside (Pn-3G) and malvidin-3-glucoside (Mv-3G) depletion (B); and for malvidin-3-O-acetylglucoside (Mv-3acG) and malvidin-3-O-coumaroylglucoside (Mv-3cmG) depletion (C) [85].
Indeed, it not only affects the kinetics of degradation reactions [86], but also the level of dissolved oxygen in wine [83,209]. Literature data suggest that optimization of the storage temperature may accelerate the ageing of wine as well as change the chemical composition of the wines, thus highlighting the sensitivity of the reaction mechanisms and the activation energy of Arrhenius to temperature changes [87,88]. Indeed, as the temperature increases from 5 to 35 °C, the oxygen saturation points of the wine decreases from 10,5 mg/L to 5,6 mg/L. However, the formation rate of quinone increases with increasing temperature [209]. Temperature beside affecting the kinetics of oxidative reactions, acting as an inhibitor, also influence the balance of sulphur dioxide (SO2) in wine [34], an important molecule distinguished as a primary preservative in vinification [89], essential for ensuring oxidation stability [90].
1.3. Effect of light
Light, particularly UV-light, is widely recognized for its role in triggering oxidation and the generation of reactive oxidative species [91]. Similar to various other beverages and food items, light accelerates oxidation in wine, promoting its overall oxidation process [34]. In the context of wine, iron-induced ionization under light exposure is suggested to primarily catalyze oxidation, since it is significant involved in the chemical oxidation process [46].Notably, iron-tartrate complexes, recognized for their contribution to oxidation, have been shown to notably enhance browning [92]. This can lead to the characteristic attributes of over-oxidized wines, featuring diminished aromatic compounds [42]; [93], browning [48], and the emergence of oxidative aromas such as acetaldehyde, acetic acid, and sotolon, which have been noted in wines subjected to light exposure [95].
1.4. Effect of closure
Once the maturation process is complete, generally, the wine is transferred into glass bottles of various capacities, which typically hold 0.75 L of wine [96]. The relationship between the size of the container and the volume of the wine is of significant importance, as it affects both the wine resistance to oxidation and the presence of gaseous elements in the headspace of the bottle [97]. Although it is possible to store wine in plastic or plastic/carton (Bag in Box) containers, glass remains the best choice for packaging [98]. Thanks to its hermetic nature, in fact, glass allows oxygen to permeate exclusively through the cork [99], and thus, the choice of the cap material plays a key role in regulating the transfer of oxygen to the bottled wine, as the permeability of the cap directly influences this process [100,101]. Cork typically exhibits gas permeability, which can facilitate the dissipation of these gases out of the bottle, acting as a barrier to various gases, including alcohol or water vapor from wine (Fig. 6).
Fig. 6.
Ingress the oxygen in the bottle and the characteristics of caps [46].
Oxygen interacts with the wine at different stages of winemaking and bottling [46], which suggests that the wine already contains dissolved oxygen at the time of bottling. In addition, oxygen remains present in the headspace after bottling [16,102], which, however, could be removed by vacuum evaporation and replaced with an inert gas, such as nitrogen or argon, in order to better manage storage and ageing.
However, the penetration of the oxygen in bottle through the stopper could represent an important factor when bottle ageing is desired to enhance the flavour and aroma of the wine [46] and the amount of oxygen able to penetrate inside the bottle can be defined as TPO (Total Oxygen Package) [103] (Fig. 5).
Fig. 5.
Oxygen input in the bottle (TPO).
A great number of studies evidenced that also the stopper characteristics greatly influence the bottle ageing process and preservation of the wine [3,100,104], as
the type of used cap that is able to modulate the oxygen ingress in the bottle (Fig. 6) [46].
For this reason, over the years, winegrowers have examined the effectiveness of various enclosures and physical modifications able to improve their sealing capabilities. The traditional sealing mechanism includes corks, which however, can be subjected to various treatments that alter its structural configuration and particle size, resulting in different oxygen permeability [46]. Wineries also relies on alternative materials for bottle closures, such as synthetic compounds, aluminium screw caps laminated with thermoplastics, or even caps made exclusively of polyethylene [100]. Nevertheless, porous closures remain the most employed method as they facilitate proper ageing of the wine, Conversely, screw caps offer a nearly hermetic closure, significantly limiting oxygen ingress that may alters the chemical environment of the wine, producing reduced qualities [105]. In contrast, polyethylene stoppers prove to be excessively porous, resulting in premature oxidation of the wine [106]. Stoppers made from permeable materials (such as cork or synthetic materials) require mechanical compression to further decrease their permeability at the interface with the glass, while not altering the overall permeability of the stopper [107].
The transference of gases such as diffusion coefficient or permeability [46] may be addressed by several methods and measures, even though, the most used and practical one is represented by OTR (Oxygen transfer rate) value [[108], [109], [110], [111]]. OTR may be calculated by physical measures of the stopper properties, i.e., by assuming their effective diffusion, by determining the oxygen concentration in the bottle, the degradation rate of compounds in the wine, or even apparent characteristics, such as yellow colour measuring absorbance at 420 nm or chemiluminescence [110,112]. The units of OTR are usually expressed as mg or ml of O2 per day, month, or year [101,113].
Since cork is a natural material, it features heterogeneous characters, thus showing a broad spectrum of OTR [46]. Agglomerated corks, more homogeneous than the natural ones, have a closer range of OTR [114], that is even tighter for the synthetic caps (from 0,003 to 0,008 cc/day/closure bottle) [26]. OTR values may still differ among the different stoppers, as the permeability of the material is still connected to its microscopic structure. In summary, evidences suggest that in general terms, natural cork stoppers have a variable but favourable OTR that may be homogenized by micro agglomeration, while synthetic stoppers offer in many cases too high OTR for long-ageing wines [46]. On the other hand, while screw caps may be a good option to preserve wine in non-optimal conditions of storage, in view also of avoiding the corky smell caused by 2,4,6-trichloroanisole (TCA) [115], tend to induce the formation of “reductive” characters [88].
1.5. Human health and wine oxidation
The factors influencing wine oxidation, therefore, are many and varied, causing not only a loss of the product desired organoleptic characteristics but also a depletion of the phenolic compounds that are extremely important bioactive molecules that beside playing a key role during vinification due to their antioxidant properties, are responsible for the nutraceutical value of the wine [116]. Indeed, in recent years, several studies have indicated the relation between moderate red wine consumption and human health (Table 1), mainly attributable to polyphenols content. However, the oxidation of the product could result in the oxidation of polyphenols and thus in the loss of all these health benefits.
Table 1.
Health benefits of moderate consumption of red wine.
| Diseases | Positive effects | References |
|---|---|---|
| Heart disease | Prevents platelet aggregation | [117] |
| Oncological diseases | Reduces esophageal cancer, reduced the progression of malignant phase of cancer, inhibited carcinogenesis with pleiotropic effect, in vivo hepatoprotective effects, suppresses the proliferation of anchorage-independent b(4) production |
[118,119] |
| Type 2 diabetes | Lowers glucose levels | [120,121] |
| Inflammatory processes | Inhibits phosphorylation activation, prevents radical formation and their activities, prevents aortic lipid deposition |
[122] |
| Oxidative stress | Prevents radical formation | [123,124] |
| Allergic diseases | Inhibits immunoglobulin (IgE) synthesis, activate of mast cells and basophils or other inflammatory cells, produce inflammatory mediators, including cytokines |
[125] |
| Neurodegenerative diseases | Neuroprotection | [[126], [127], [128]] |
2. Prevention of oxidation in wine
2.1. Sulphur dioxide
To safeguard wines from oxidation, sulphur dioxide (SO2), whose main properties are reported in Table 2, has been utilized since the 18th century, primarily for its antiseptic properties, as well as for its activity as an inhibitor of enzymatic and chemical oxidation, thereby aiding in reducing the browning rate [129,130].
Table 2.
Properties of sulphur dioxide.
| Properties | Advantages | Disadvantages | References |
|---|---|---|---|
| Antioxidant | Antioxidase activity, reduce quinones formation, reaction with hydrogen peroxide | Effectiveness depends on pH | [11,145,146] |
| Antimicrobial | Prevents unintentional fermentations, prevents formation of off-flavours | Effectiveness depend on pH | [[147], [148], [149], [150], [151], [152]] |
| Dissolving | Favourite extraction of phenolic compounds | No significant colour improvement | [145] |
| Sensory | Wines treated with SO2 exhibit a more intricate flavour profile, particularly evident in instances involving overripe or subpar grape varieties | An excessive use of SO2 cause formation of mercaptans and hydrogen sulphide | [145,153] |
| Health | Allergic reactions in some people | [[135], [136], [137], [138], [139], [140], [141], [142], [143]] |
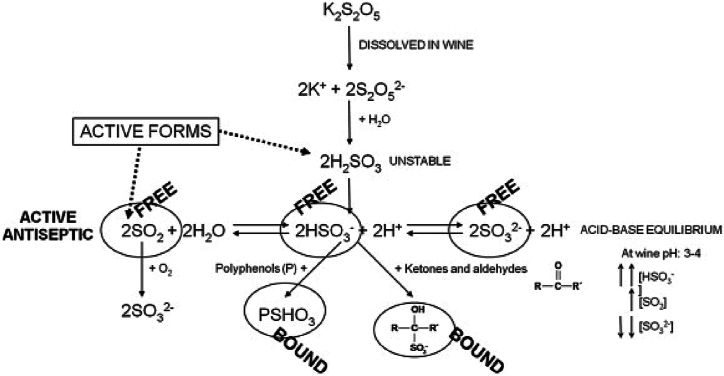
Generally, sulphur dioxide is added to wine as potassium metabisulfite (K2S2O5) and, at the wine pH, exists both as molecular form as SO2, and, more commonly, (94–99 % at wine pH) as bisulphite ion, HSO3− [22]. Once dissolved in wine, sulphur dioxide bind various wine constituents such as acetaldehyde, anthocyanins, pyruvic acid, glutaric acid, glucose, or specific phenolic compounds. Notably, ethanal, pyruvic acid, and 2-oxoglutaric acid exhibit particular reactivity with sulphur dioxide [131]. Indeed, there are two fractions of SO2 in wine, the "free SO2," comprising HSO3− and SO2, and the "bound SO2" that primarily indicating sulphur dioxide bound to unsaturated compounds [132,133]. Only free SO2 effectively combats oxidation (Fig. 7), even though, below 10 mg L−1 of free SO2 in wine, this protective effect diminishes [129].
Fig. 7.
Chemical balance of SO2 added to wine as potassium metabisulphite [132].
Sulphur dioxide in wine makes a key role against oxidation by interacting with hydrogen peroxide, rather than the direct oxygen scavenging mechanism [76]. Thus, sulphur dioxide can avoid the formation of aldehydes by competing for hydrogen peroxide [134]. Nevertheless, sulphur dioxide may lead to health issues [[135], [136], [137], [138], [139], [140], [141], [142], [143]], and an increasing number of consumers exhibit allergies, intolerances, and sensitivities associated with wine consumption. According to a study published in a German scientific journal, 'wine intolerance' affects 8.9 % of women and 5.2 % of men, while in the United States, approximately 12 % of women and 8 % of men are affected due to heightened exposure to synthetic chemicals from food and the environment [144]. Several studies confirmed the toxicity of sulphur dioxide by inhalation, showing corrosion and irritation of the respiratory tract. Moreover, the assumption of sulphur dioxide is related to changes in the metabolism of certain amino acids and vitamin B1. The mechanism behind this problem is linked to the antivitamin action of sulphites against thiamine (vitamin B1), whose deficiency causes significant changes in sugar metabolism, such as diabetes.
In the body, sulphites (SO₃2⁻) are converted into sulphates (SO₄2⁻) at digestive apparatus level and the reaction is catalyzed by a molybdenum-containing hemoprotein called sulphite oxidase. During this phase, sulphites release sulphur dioxide, causing gastric irritation and pain accompanied by vomiting if the ingested dose exceeds 3.5 mg/kg of body weight (acute poisoning).
Allergic reactions to sulphites are manifested by the intake of very low doses (of the order of the milligram) and mainly concern asthmatic subjects. Although sensitivity to sulphites is not clearly demonstrated for non-asthmatic subjects, the concern of potential allergic reactions has induced the FDA (Food and Drug Administration) in the United States to make mandatory the indication of the presence of sulphites on labels when their concentration exceeds 10 mg/L [145].
Therefore, the WHO (World Health Organization) has established the Acceptable Daily Intake (ADI) corresponding to 0.7 mg of SO2 per kg of body weight. However, the amount of sulphur actually assimilated by the consumer is often underestimated, since sulphur dioxide and its derivatives are widely used as additives, not only in the winemaking sector, but also in many foods. For this reason, the European Regulation (EU Regulations 606/2009 and 479/2008) establishes the maximum doses of total SO2 of 150 mg/L for red wines and 200 mg/L for white wines, rosé or rosé, and in wines containing a residual sugar of 5 g/L.
2.1.1. The inhibition, activation, and selection of yeasts and bacteria
SO2 serves as a potent inhibitor of various microorganisms, including yeasts, lactic acid bacteria (LAB), and to a lesser extent, acetic acid bacteria. Its antimicrobial action effectively prevents yeast haze formation, undesirable secondary fermentation, growth of Brettanomyces, proliferation of mycodermic yeasts, and various types of bacterial spoilage [151]. However, it is important to note that the SO2 effectiveness is inversely proportional to the wine pH as it decreases as the wine pH increases (Fig. 4), making the microbiological stabilization of wines with low acidity challenging.
In winemaking, SO2 is commonly employed to control the LAB growth and the development of malolactic fermentation (MLF), primarily due to its selective antimicrobial properties, especially against LAB [154]. The three primary genera of LAB associated with winemaking are Oenococcus, Pediococcus, and Lactobacillus. Among these, Oenococcus oeni is particularly well-adapted to the harsh conditions of winemaking, making it the primary species responsible for MLF in wine [155]. Other LAB species, such as Lactobacillus hilgardii and Pediococcus pentosaceus, can negatively impact wine quality by causing alterations such as "lactic disease" and the production of off-flavor compounds [147], as well as biogenic amines [148]. Lactobacillus bacteria, especially L. hilgardii, are responsible for the phenomenon known as "piqûre lactique," wherein they utilize residual sugars in slow alcoholic fermentations, leading to an increase in volatile acidity and modification of the wine sensory properties [132,150].
Concerning yeasts, species of Brettanomyces genus, along with their sporulating form, contribute to the development of bad odours in wine described as "leather," "animal," and "horse sweat," primarily due to the production of ethyl-phenols from grape hydroxycinnamic acids. The presence of Brettanomyces in barrels, often due to inadequate cleaning, is a common cause of off-flavor development [132,152].
SO2 effectively combats these microorganisms at concentrations typically used in winemaking. Therefore, replacing SO2 is expected to assist to a control of these microorganisms throughout the winemaking process [132].
2.2. Other alternative additives
Red wines show different antioxidant activity [145], related to grape cultivar and terroir, production methods, extraction techniques, and oxygen exposure. Usually sulphur dioxide is added to wine playing a key role due to its various protective capacities towards wine [93]. However, since it is responsible for some drawbacks, some efforts to reduce and/or remove it have been made, mainly evaluating additives and/or alternative practices, discussed below, to be employed in winemaking as follows.
2.2.1. Lysozime
Lysozyme, a 129-amino acid protein isolated from egg albumen, has demonstrated to be an effective antimicrobial agent in various food products [156]; [94]. Lysozyme offers potential advantages over SO2 [157]. Indeed, depending on the timing of its addition, it may marginally enhance the colour of red wines by avoiding bleaching effects. However, its application in red wine determines several drawbacks, including the extraction and presence of phenolic compounds, notably low molecular weight proanthocyanidins, which hinder its efficacy in red wine production [158].
2.2.2. Ascorbic acid
Ascorbic acid serves as an additional antioxidant employed in winemaking. It is widely recognized for its ability to scavenge [158] molecular oxygen prior to the oxidation of phenolic compounds particularly in white wines, although it is typically combined with SO2 to neutralize the hydrogen peroxide generated during the oxidation of ascorbic acid [158]. However, a notable issue determined by the addition of ascorbic acid is represented by the tendency to induce browning during storage [159].
2.2.3. Glutathione
Glutathione has been suggested as an alternative to reduce SO2 levels because of its antioxidant properties, although its significance lies primarily in white wine production rather than red wine [159]. However, glutathione is most effective in combination with sulphur dioxide, thus allowing a reduction but not the elimination of SO2 [160,161].
2.2.4. Dimethyl decarbonate
Dimethyl dicarbonate (DMDC) is a microbial inhibitor that has been suggested as an alternative to SO2 in winemaking [161,162]. It has been authorized for use in wines by the European Union, with a maximum eligible amount of 200 mg/L per bottle for wines containing more than 5 g/L of residual sugar (Regulation (EC) No 643/2006) [160]. Similarly, in the USA, it can be employed during wine storage in regular doses up to 200 mg/L [163].
In wine, DMDC is rapidly converted to methanol, with the formation of minor quantities of methylcarbonate and alkylcarbonates due to interactions between DMDC and polyphenols or organic acids. Researchers have indicated that the methanol concentration resulting from DMDC addition does not reach toxicologically significant levels [162].
2.2.5. Bacteriocin
Bacteriocins, such as nisin, pediocin, and plantaricin, are small polypeptides synthesized by specific LAB, exhibiting inhibitory effects on other bacterial species [164]. Notably, some of these compounds are legally permitted as food additives. Researchers indicate that they primarily target the cytoplasmic membrane of gram-positive bacteria, leading to an increased permeability to small ionic components and eventual cell lysis [165]. However, their efficacy against gram-negative bacteria varies depending on the species [165,166]. Due to their lack of colour or odour and non-toxic nature, bacteriocins are considered ideal preservatives against gram-positive bacteria [167]. Moreover, they exhibit high specificity, selectively targeting certain groups of microorganisms. Combining these compounds with metabisulfite has been suggested as a strategy to control the proliferation of spoilage bacteria in wine, potentially allowing a reduction in the levels of sulphur dioxide currently employed by the wine industry [168].
2.2.6. Yest less
Another conventional method for safeguarding wine from oxidation involves the utilization of yeast lees [169]. Fresh lees exhibit a notable capacity for oxygen absorption owing to the presence of lipids and sterols of yeast membranes [170], along with polyphenols, thiol groups found in cell wall proteins [171], and β-glucans derived from yeast cell walls [172], all contributing to their antioxidative characteristics. Nevertheless, prolonged contact with lees may alter the sensory attributes of wine; thus, it may not be suitable for all wine varieties. Additionally, relying solely on lees does not shield wine from microbial contamination, necessitating the use of sulphites [162].
2.2.7. Colloidal silver complex
Silver has long been recognized for its antimicrobial properties, dating back to ancient times, even though the mechanisms of action were not fully understood [173]. However, the use of silver for its antimicrobial effects declined with the advent of antibiotics consecutive to the discovery of penicillin [174]. Nevertheless, as biocide-resistant bacterial strains emerged, interest in silver, particularly silver nanoparticles, as an antimicrobial agent resurged [174]. Some researches indicate the ability of silver nanomaterials to exhibit antimicrobial activity against a wide range of both Gram-negative and Gram-positive bacteria, as well as some antifungal and antiviral properties [175,176]. The three primary mechanisms of action of silver include the absorption of free silver ions leading to disruption of ATP production and DNA replication [175], the generation of reactive oxygen species (ROS) by silver nanoparticles and silver ions [175], and direct damage to cell membranes by silver nanoparticles [175,176]. In the food industry, silver nanomaterials are being employed for water purification [177], for the development of novel antimicrobial packaging materials [178], and for replacing SO2 in wine [175].
2.2.8. Pulsed electric fields
The method known as pulsed electric fields (PEF) presents a rapid, non-thermal, and remarkably effective approach to eliminate pathogenic microorganisms in food products while preserving their quality [179,180]. In this technique, short pulses of high electric field voltage (up to 70 kV/cm), typically lasting microseconds, are applied to food placed between two electrodes [[180], [181], [182]]. These high-voltage pulses induce electroporation of cell membranes, exceeding the natural membrane potential and thus increasing their permeability [181]. When this difference in the membrane potential exceeds the critical threshold of about 1 V between the outer and inner membrane, polarisation occurs, leading to membrane rupture. A sufficiently high field voltage and pulse duration, determine irreversible membrane damage of vegetative microorganisms, resulting in their deactivation [183]. Furthermore, Garde-Cerda′n et al. [146], showed that the treatment of wine must with PEF allows a reduction in SO2 concentration.
2.2.9. UV
UV irradiation has emerged as a non-thermal method for water disinfection, which has proved to be very effective in eliminating microbial contaminants from surfaces and packaging in the food industry [184]. In particular, UV-C radiations have found application in food processing to deactivate various microorganisms, including bacteria and yeasts, as well as enzymes such as PPO (poliphenoloxidases), in a wide range of liquid products [137,184,185].
The potential of UV irradiation as an alternative approach to wine preservation was explored in a study by Fredericks et al., in 2011 [137]. Furthermore, it has been observed that grapes subjected to abiotic stress through post-harvest UV-C treatment exhibit greater levels of stilbenes [186]. This discovery paves the way for a new winemaking technique aiming at producing white wine enriched with resveratrol [187,188], a compound known for its various bioactive properties, including anti-cancer, anti-inflammatory, glycaemic-regulating and cardiovascular [188].
2.2.10. High pressure
High pressure (HP) is a non-thermal method used in food processing subjecting products to instantaneous and uniform pressures ranging from 100 to 1000 MPa [189], whereas the product size and geometry [189,190]. Considering green technologies, HP employs water as a compression medium and is highly energy-efficient [191]. Over the past decade, the use of HP technology for the production of microbiologically safe foods has significantly increased in the industry. Currently, most industrial HP equipment used in food processing operates on a batch processing basis.
This technology is widely used for microbial [192,193] and enzyme [194,195] inactivation and also for modifying the functional properties of certain food components [196,197]. Microbial inactivation by HP is primarily attributed to disruptions in cellular structures and functions, such as membranes, ribosomes, and enzymes [198], leading to cell leakage. Since HP affects non-covalent bonds while preserving covalent bonds [197], foods treated with HP retain their original freshness, flavour, taste, and colour. Small molecules such as volatile compounds, pigments, vitamins, and other compounds responsible for sensory, nutritional, and health-promoting benefits are largely retained after HP treatment [189].
For instance, studies have shown that HP treatments ranging from 400 to 600 MPa at room temperature can inactivate polyphenoloxidase (PPO), and β-glucosidase enzymes while preserving monomeric and polymeric anthocyanins and individual phenolic compounds in strawberry pulp [189]. In the oenological sector, HP treatments have been tested to preserve the quality and sustainability of grape juice and must [[199], [200], [201]], as well as wine [[202], [203], [204]]. Researchers have demonstrated that pressure treatments of 400 MPa for 2 min at 20 °C effectively is able to microorganisms added to wine, such as Oenococcus oeni, Lactobacillus spp., Acetobacter, and Botrytis cinerea [203]. Similarly, pressure treatments of 500 MPa for 5 min significantly reduce the initial microbial wine population without altering the physicochemical or organoleptic properties of the wine [204]. Some studies have shown that microbial inactivation increases with higher pressure treatments and longer durations, with aerobic bacteria being more susceptible to HP treatments than yeasts and LAB [203].
Therefore, the use of HP for wine preservation is currently only feasible in the final stage of winemaking, replacing the addition of SO2 before bottling with a pressure treatment after bottling [151] and not for entire winemaking processes [199,205].
3. Future perspectives for extending wine shelf life
Future perspectives in wine packaging emphasize the development of smart packaging technologies to extend product shelf life. These innovations primarily deal with the oxidation prevention, thereby preserving the wine quality [38]. Additionally, there is a significant notable shift towards environmentally friendly solutions, reducing the carbon footprint associated with traditional packaging methods. By integrating advanced materials and sustainable practices, wine industry may enhance product longevity while addressing environmental concerns [206].
For example, the importance of storage conditions and type of packaging in preventing oxidation is emphasised by factors such as the presence of antioxidants, oxygen levels in the packaging and storage temperature. This indicates the need for careful control of these variables in wine packaging [207]. Innovations such as the use of oxygen scavengers, modified atmosphere packaging, and the development of biodegradable and recyclable materials demonstrate the industry interest for extending shelf life while being mindful of environmental impact [208]. By adopting these advanced packaging solutions, the wine industry can ensure that wines maintain their quality over time and contribute to a more sustainable future.
4. Conclusion
In conclusion, the concept of wine shelf life is multifaceted and includes factors such as retention of initial flavours, progression to ideal character, and commercial acceptability of the wine. The ageing potential of the wine plays a significant role, with some wines developing an aged bouquet over time. However, factors such as oxidation, influenced by exposure to oxygen, temperature, and light, my have a significant impact on wine quality and shelf life. Oxidative reactions, particularly non-enzymatic oxidation reactions, catalyzed by metal ions such as iron, contribute to colour changes and flavour degradation in wine. Temperature fluctuations affect the speed of chemical reactions, including oxidation, while exposure to light, particularly UV light, accelerates oxidation processes. The choice of closure also influences wine preservation, with porous stoppers allowing controlled oxygen transfer, while screw caps offer nearly hermetic seals. The transference of gases, particularly oxygen, is a critical consideration in determining closure effectiveness, with factors such as diffusion coefficients and oxygen transfer rates influencing closure selection. Natural cork stoppers exhibit varying oxygen transfer rates, while synthetic stoppers may offer excessive rates for long-ageing wines. Overall, understanding and managing these factors are essential for preserving wine quality and extending its shelf life.
Funding
This research was funded by TRACEWINDU (Traceability at wine industry through inte- 402 grated labelling of typicality, health protection effect and organoleptic attributes), project co- 403 founded by Horizon 2020 Framework Programme of European Union under the Grant Agreement 404 no 101007979 running from 1 June 2021 to 31 May 2025.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CRediT authorship contribution statement
Nicola Mercanti: Writing – review & editing, Writing – original draft, Visualization, Validation, Software, Methodology, Investigation, Formal analysis, Data curation. Monica Macaluso: Writing – review & editing, Writing – original draft, Resources. Ylenia Pieracci: Writing – review & editing, Writing – original draft, Visualization, Investigation, Data curation. Francesco Brazzarola: Validation, Supervision. Fabrizio Palla: Supervision, Conceptualization. Piero Giorgio Verdini: Validation, Supervision. Angela Zinnai: Supervision, Resources, Project administration, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:Ylenia Pieracci reports financial support was provided by University of Pisa, Department of Agriculture, Food and Environment. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Abedi‐Firoozjah R., Salim S.A., Hasanvand S., Assadpour E., Azizi‐Lalabadi M., Prieto M.A., Jafari S.M. Application of smart packaging for seafood: a comprehensive review. Compr. Rev. Food Sci. Food Saf. 2023;22:1438–1461. doi: 10.1111/1541-4337.13117. [DOI] [PubMed] [Google Scholar]
- 2.Tanner D. Reference Module in Food Science. Elsevier; 2016. Impacts of storage on food quality. B978008100596503479X. [DOI] [Google Scholar]
- 3.Oliveira A.S., Furtado I., Bastos M.D.L., Guedes De Pinho P., Pinto J. The influence of different closures on volatile composition of a white wine. Food Packag. Shelf Life. 2020;23 doi: 10.1016/j.fpsl.2020.100465. [DOI] [Google Scholar]
- 4.Vakili-Ghartavol M., Arouiee H., Golmohammadzadeh S., Naseri M., Bandian L. Edible coatings based on solid lipid nanoparticles containing essential oil to improve antimicrobial activity, shelf-life, and quality of strawberries. J. Stored Prod. Res. 2024;106 doi: 10.1016/j.jspr.2024.102262. [DOI] [Google Scholar]
- 5.Amani P., Gadde L.-E., Amani P., Gadde L.-E. Shelf life extension and food waste reduction. 2015. [DOI]
- 6.Cirillo G., Spizzirri U.G., Iemma F., editors. Functional Polymers in Food Science: from Technology to Biology. first ed. Wiley; 2015. [DOI] [Google Scholar]
- 7.Fadiji T., Rashvand M., Daramola M.O., Iwarere S.A. A review on antimicrobial packaging for extending the shelf life of food. Processes. 2023;11:590. doi: 10.3390/pr11020590. [DOI] [Google Scholar]
- 8.Jiang G., Hou X., Zeng X., Zhang C., Wu H., Shen G., Li S., Luo Q., Li M., Liu X., Chen A., Wang Z., Zhang Z. Preparation and characterization of indicator films from carboxymethyl-cellulose/starch and purple sweet potato (Ipomoea batatas (L.) lam) anthocyanins for monitoring fish freshness. Int. J. Biol. Macromol. 2020;143:359–372. doi: 10.1016/j.ijbiomac.2019.12.024. [DOI] [PubMed] [Google Scholar]
- 9.Khan A., Ezati P., Rhim J.-W. Chitosan/gelatin-based multifunctional film integrated with green tea carbon dots to extend the shelf life of pork. Food Packag. Shelf Life. 2023;37 doi: 10.1016/j.fpsl.2023.101075. [DOI] [Google Scholar]
- 10.Muñoz-Bernal Ó.A., De La Rosa L.A., Rodrigo-García J., Martínez-Ruiz N.R., Sáyago-Ayerdi S., Rodriguez L., Fuentes E., Alvarez-Parrilla E. Phytochemical characterization and antiplatelet activity of Mexican red wines and their by-products. South Afr. J. Enol. Vitic. 2021;42 doi: 10.21548/42-1-4450. [DOI] [Google Scholar]
- 11.Oliveira C.M., Ferreira A.C.S., De Freitas V., Silva A.M.S. Oxidation mechanisms occurring in wines. Food Res. Int. 2011;44:1115–1126. doi: 10.1016/j.foodres.2011.03.050. [DOI] [Google Scholar]
- 12.Castellari M., Matricardi L., Arfelli G., Galassi S., Amati A. Level of single bioactive phenolics in red wine as a function of the oxygen supplied during storage. Food Chem. 2000;69:61–67. doi: 10.1016/S0308-8146(99)00240-X. [DOI] [Google Scholar]
- 13.De Beer D., Joubert E., Marais J., Manley M. Colour and Sensory Quality of Pinotage Wine. 2008. Effect of oxygenation during maturation on phenolic composition, total antioxidant capacity. [Google Scholar]
- 14.Zafrilla P., Morillas J., Mulero J., Cayuela J.M., Martínez-Cachá A., Pardo F., López Nicolás J.M. Changes during storage in conventional and ecological wine: phenolic content and antioxidant activity. J. Agric. Food Chem. 2003;51:4694–4700. doi: 10.1021/jf021251p. [DOI] [PubMed] [Google Scholar]
- 15.Odriozola-Serrano I., Oms-Oliu G., Soliva-Fortuny R., Martín-Belloso O. Effect of high-oxygen atmospheres on the antioxidant potential of fresh-cut tomatoes. J. Agric. Food Chem. 2009;57:6603–6610. doi: 10.1021/jf900776j. [DOI] [PubMed] [Google Scholar]
- 16.Tarko T., Duda-Chodak A., Sroka P., Siuta M. The impact of oxygen at various stages of vinification on the chemical composition and the antioxidant and sensory properties of white and red wines. Int. J. Food Sci. 2020;2020:1–11. doi: 10.1155/2020/7902974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cv U., Ke A., Co N., Pc I., Cs U., Li U., Ns O. Effects of selected plant preservatives on microbial load and shelf life of palm wine. Asian J. Food Res. Nutr. 2024;3:85–91. [Google Scholar]
- 18.De Lima G.S., Araújo A.S., Berger L.R.R., Fai A.E.C., Barbosa De Lima M.A., França R., Stamford T.C.M. Chitosan: Novel Applications in Food Systems. Elsevier; 2023. Chitosan as an antimicrobial agent to increase shelf life of foods; pp. 155–191. [DOI] [Google Scholar]
- 19.Wirth J., Morel-Salmi C., Souquet J.M., Dieval J.B., Aagaard O., Vidal S., Fulcrand H., Cheynier V. The impact of oxygen exposure before and after bottling on the polyphenolic composition of red wines. Food Chem. 2010;123:107–116. doi: 10.1016/j.foodchem.2010.04.008. [DOI] [Google Scholar]
- 20.Jackson R. The Stability and Shelf Life of Food. Elsevier; 2016. Shelf life of wine; pp. 311–346. [DOI] [Google Scholar]
- 21.Arias-Pérez I., Sáenz-Navajas M.P., de-la-Fuente-Blanco A., Ferreira V., Escudero A. Insights on the role of acetaldehyde and other aldehydes in the odour and tactile nasal perception of red wine. Food Chem. 2021;361 doi: 10.1016/j.foodchem.2021.130081. [DOI] [PubMed] [Google Scholar]
- 22.Dalton D.R. The Chemistry of Wine. Oxford University Press; 2018. Specialized wines. [DOI] [Google Scholar]
- 23.Forino M., Picariello L., Lopatriello A., Moio L., Gambuti A. New insights into the chemical bases of wine color evolution and stability: the key role of acetaldehyde. Eur. Food Res. Technol. 2020;246:733–743. doi: 10.1007/s00217-020-03442-x. [DOI] [Google Scholar]
- 24.To N.D.K., Theruvathu J.A. Determination and quantification of acetaldehyde, acetone, and methanol in hand sanitizers using headspace GC/MS: effect of storage time and temperature. Int. J. Environ. Res. Publ. Health. 2024;21:74. doi: 10.3390/ijerph21010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Medina-Plaza C., DuBois A., Tomasino E., Oberholster A. Effect of storing conditions (lighting, temperature and bottle color) on rosé wine attributes. Food Chem. 2024;439 doi: 10.1016/j.foodchem.2023.138032. [DOI] [PubMed] [Google Scholar]
- 26.Silva M.A., Julien M., Jourdes M., Teissedre P.-L. Impact of closures on wine post-bottling development: a review. Eur. Food Res. Technol. 2011;233:905–914. doi: 10.1007/s00217-011-1603-9. [DOI] [Google Scholar]
- 27.Lambrechts M.G., Pretorius I.S. Yeast and its importance to wine aroma - a review. South Afr. J. Enol. Vitic. 2019;21 doi: 10.21548/21-1-3560. [DOI] [Google Scholar]
- 28.Pastor-Vega N., Carbonero-Pacheco J., Mauricio J.C., Moreno J., García-Martínez T., Nitin N., Ogawa M., Rai R., Moreno-García J. Flor yeast immobilization in microbial biocapsules for Sherry wine production: microvinification approach. World J. Microbiol. Biotechnol. 2023;39:271. doi: 10.1007/s11274-023-03713-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zea L., Serratosa M.P., Mérida J., Moyano L. Acetaldehyde as key compound for the authenticity of sherry wines: a study covering 5 decades. Compr. Rev. Food Sci. Food Saf. 2015;14:681–693. doi: 10.1111/1541-4337.12159. [DOI] [Google Scholar]
- 30.He Y., Wang X., Li P., Lv Y., Nan H., Wen L., Wang Z. Research progress of wine aroma components: a critical review. Food Chem. 2023;402 doi: 10.1016/j.foodchem.2022.134491. [DOI] [PubMed] [Google Scholar]
- 31.Giovanelli G., Brenna O.V. Oxidative stability of red wine stored in packages with different oxygen permeability. Eur. Food Res. Technol. 2007;226:169–179. doi: 10.1007/s00217-006-0523-6. [DOI] [Google Scholar]
- 32.Karbowiak T., Gougeon R.D., Alinc J.-B., Brachais L., Debeaufort F., Voilley A., Chassagne D. Wine oxidation and the role of cork. Crit. Rev. Food Sci. Nutr. 2009;50:20–52. doi: 10.1080/10408390802248585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee D.-H., Kang B.-S., Park H.-J. Effect of oxygen on volatile and sensory characteristics of cabernet sauvignon during secondary shelf life. J. Agric. Food Chem. 2011;59:11657–11666. doi: 10.1021/jf200759d. [DOI] [PubMed] [Google Scholar]
- 34.Scrimgeour N., Nordestgaard S., Lloyd N.D.R., Wilkes E.N. Exploring the effect of elevated storage temperature on wine composition: elevated storage temperature and wine composition. Aust. J. Grape Wine Res. 2015;21:713–722. doi: 10.1111/ajgw.12196. [DOI] [Google Scholar]
- 35.Fracassetti D., Limbo S., Pellegrino L., Tirelli A. Il difetto di luce nel vino bianco: Effetto ed evoluzione nel corso della conservazione. BIO Web Conf. 2019;15 doi: 10.1051/bioconf/20191502028. [DOI] [Google Scholar]
- 36.Lan H., Li S., Yang J., Li J., Yuan C., Guo A. Effects of light exposure on chemical and sensory properties of storing Meili Rosé wine in colored bottles. Food Chem. 2021;345 doi: 10.1016/j.foodchem.2020.128854. [DOI] [PubMed] [Google Scholar]
- 37.Laposa Z., Vesztergom S., Kocsis M., Keszei E. In situ measurement of light transmission into wine bottles and calculation of shelf life. OENO One. 2023;57:265–277. doi: 10.20870/oeno-one.2023.57.1.7172. [DOI] [Google Scholar]
- 38.Kanavouras A., Karanika E., Coutelieris F.A., Kotseridis Y., Kallithraka S. Preliminary study of flavor compounds as oxidation markers in bottled white wines of Greek origin. OENO One. 2019;53 doi: 10.20870/oeno-one.2019.53.3.2439. [DOI] [Google Scholar]
- 39.Pinto J., Oliveira A.S., Azevedo J., De Freitas V., Lopes P., Roseira I., Cabral M., Guedes De Pinho P. Assessment of oxidation compounds in oaked Chardonnay wines: a GC–MS and 1 H NMR metabolomics approach. Food Chem. 2018;257:120–127. doi: 10.1016/j.foodchem.2018.02.156. [DOI] [PubMed] [Google Scholar]
- 40.Bekker M.Z., Day M.P., Holt H., Wilkes E., Smith P.A. Effect of oxygen exposure during fermentation on volatile sulfur compounds in Shiraz wine and a comparison of strategies for remediation of reductive character: macro-oxygenation: effect on sulfurous off-odours. Aust. J. Grape Wine Res. 2016;22:24–35. doi: 10.1111/ajgw.12172. [DOI] [Google Scholar]
- 41.Picariello L., Rinaldi A., Forino M., Errichiello F., Moio L., Gambuti A. Effect of different enological tannins on oxygen consumption, phenolic compounds, color and astringency evolution of aglianico wine. Molecules. 2020;25:4607. doi: 10.3390/molecules25204607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ugliano M. Oxygen contribution to wine aroma evolution during bottle aging. J. Agric. Food Chem. 2013;61:6125–6136. doi: 10.1021/jf400810v. [DOI] [PubMed] [Google Scholar]
- 43.Benucci I., Cerreti M., Esti M. Dosing oxygen from the early stages of white winemaking: effect on oxidation–reduction potential, browning stability, volatile composition, and sensory properties. Food Chem. 2024;432 doi: 10.1016/j.foodchem.2023.137243. [DOI] [PubMed] [Google Scholar]
- 44.Tedesco I., Spagnuolo C., Russo G.L., Russo M., Cervellera C., Moccia S. The pro-oxidant activity of red wine polyphenols induces an adaptive antioxidant response in human erythrocytes. Antioxidants. 2021;10:800. doi: 10.3390/antiox10050800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Claus H. How to deal with uninvited guests in wine: copper and copper-containing oxidases. Fermentation. 2020;6:38. doi: 10.3390/fermentation6010038. [DOI] [Google Scholar]
- 46.Echave J., Barral M., Fraga-Corral M., Prieto M.A., Simal-Gandara J. Bottle aging and storage of wines: a review. Molecules. 2021;26:713. doi: 10.3390/molecules26030713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li H., Guo A., Wang H. Mechanisms of oxidative browning of wine. Food Chem. 2008;108:1–13. doi: 10.1016/j.foodchem.2007.10.065. [DOI] [Google Scholar]
- 48.Zhao X., Duan C.-Q., Li S.-Y., Zhang X.-K., Zhai H.-Y., He F., Zhao Y.-P. Non-enzymatic browning of wine induced by monomeric flavan-3-ols: a review. Food Chem. 2023;425 doi: 10.1016/j.foodchem.2023.136420. [DOI] [PubMed] [Google Scholar]
- 49.Almohammad S.M., Naszódi M., Lángi Z. 2020. An Analogue of a Theorem of Steinitz for Ball Polyhedra in $\mathbb{R}^3$ [Google Scholar]
- 50.Vela E., Hernández-Orte P., Franco-Luesma E., Ferreira V. The effects of copper fining on the wine content in sulfur off-odors and on their evolution during accelerated anoxic storage. Food Chem. 2017;231:212–221. doi: 10.1016/j.foodchem.2017.03.125. [DOI] [PubMed] [Google Scholar]
- 51.Ferreira V., Bueno M., Franco-Luesma E., Culleré L., Fernández-Zurbano P. Key changes in wine aroma active compounds during bottle storage of Spanish red wines under different oxygen levels. J. Agric. Food Chem. 2014;62:10015–10027. doi: 10.1021/jf503089u. [DOI] [PubMed] [Google Scholar]
- 52.He F., Liang N.-N., Mu L., Pan Q.-H., Wang J., Reeves M.J., Duan C.-Q. Anthocyanins and their variation in red wines I. Monomeric anthocyanins and their color expression. Molecules. 2012;17:1571–1601. doi: 10.3390/molecules17021571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Deshaies S., Cazals G., Enjalbal C., Constantin T., Garcia F., Mouls L., Saucier C. Red wine oxidation: accelerated ageing tests, possible reaction mechanisms and application to syrah red wines. Antioxidants. 2020;9:663. doi: 10.3390/antiox9080663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Danilewicz J.C., Seccombe J.T., Whelan J. Mechanism of interaction of polyphenols, oxygen, and sulfur dioxide in model wine and wine. Am. J. Enol. Vitic. 2008;59:128–136. doi: 10.5344/ajev.2008.59.2.128. [DOI] [Google Scholar]
- 55.Fernández De Simón B., Hernández T., Cadahía E., Dueñas M., Estrella I. Phenolic compounds in a Spanish red wine aged in barrels made of Spanish, French and American oak wood. Eur. Food Res. Technol. 2003;216:150–156. doi: 10.1007/s00217-002-0637-4. [DOI] [Google Scholar]
- 56.Flamini R., Panighel A., De Marchi F. Mass spectrometry in the study of wood compounds released in the barrel‐aged wine and spirits. Mass Spectrom. Rev. 2023;42:1174–1220. doi: 10.1002/mas.21754. [DOI] [PubMed] [Google Scholar]
- 57.Sanz M., De Simón B.F., Cadahía E., Esteruelas E., Muñoz Á.M., Hernández M.T., Estrella I. Polyphenolic profile as a useful tool to identify the wood used in wine aging. Anal. Chim. Acta. 2012;732:33–45. doi: 10.1016/j.aca.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 58.Castro M.C., Silvello G.C., Corniani L.S., Acevedo M.S.M.S.F., De Andrade Marcondes Pereira A., Alcarde A.R. Maturation-related phenolic compounds in cachaça aged in oak barrels: influence of reuses. Wood Sci. Technol. 2023;57:781–795. doi: 10.1007/s00226-023-01474-6. [DOI] [Google Scholar]
- 59.Gutiérrez-Escobar R., Aliaño-González M.J., Cantos-Villar E. Wine polyphenol content and its influence on wine quality and properties: a review. Molecules. 2021;26:718. doi: 10.3390/molecules26030718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gambuti A., Siani T., Picariello L., Rinaldi A., Lisanti M.T., Ugliano M., Dieval J.B., Moio L. Oxygen exposure of tannins-rich red wines during bottle aging. Influence on phenolics and color, astringency markers and sensory attributes. Eur. Food Res. Technol. 2017;243:669–680. doi: 10.1007/s00217-016-2780-3. [DOI] [Google Scholar]
- 61.Carpena M., Pereira A.G., Prieto M.A., Simal-Gandara J. Wine aging technology: fundamental role of wood barrels. Foods. 2020;9:1160. doi: 10.3390/foods9091160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ribéreau-Gayon . fifth ed. 2004. Les phénols volatils responsables des certaines déviations olfactives de type< phénolé> des vins. [Google Scholar]
- 63.Bai Y., Zhang W., Li Y., Tan J., Han F. Glass volume or shape influence the aroma attributes of Cabernet Sauvignon dry red wine. J. Sensory Stud. 2023;38 doi: 10.1111/joss.12828. [DOI] [Google Scholar]
- 64.Castellanos E.R., Jofre V.P., Fanzone M.L., Assof M.V., Catania A.A., Diaz-Sambueza A.M., Heredia F.J., Mercado L.A. Effect of different closure types and storage temperatures on the color and sensory characteristics development of Argentinian Torrontes Riojano white wines aged in bottles. Food Control. 2021;130 doi: 10.1016/j.foodcont.2021.108343. [DOI] [Google Scholar]
- 65.Furtado I., Lopes P., Oliveira A.S., Amaro F., Bastos M.D.L., Cabral M., Guedes De Pinho P., Pinto J. The impact of different closures on the flavor composition of wines during bottle aging. Foods. 2021;10:2070. doi: 10.3390/foods10092070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pandey B., Sheth P.N., Prajapati Y.K. Air-CO2 and oxygen-enriched air-CO2 biomass gasification in an autothermal downdraft gasifier: experimental studies. Energy Convers. Manag. 2022;270 doi: 10.1016/j.enconman.2022.116216. [DOI] [Google Scholar]
- 67.Kamal A., Narasimha Rao M.P., Swapna P., Srinivasulu V., Bagul C., Shaik A.B., Mullagiri K., Kovvuri J., Reddy V.S., Vidyasagar K., Nagesh N. Synthesis of β-carboline–benzimidazole conjugates using lanthanum nitrate as a catalyst and their biological evaluation. Org. Biomol. Chem. 2014;12:2370–2387. doi: 10.1039/C3OB42236D. [DOI] [PubMed] [Google Scholar]
- 68.Apel K., Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- 69.Shaik S., Kumar D., De Visser S.P., Altun A., Thiel W. Theoretical perspective on the structure and mechanism of cytochrome P450 enzymes. Chem. Rev. 2005;105:2279–2328. doi: 10.1021/cr030722j. [DOI] [PubMed] [Google Scholar]
- 70.Singleton V.L. Oxygen with phenols and related reactions in musts, wines, and model systems: observations and practical implications. Am. J. Enol. Vitic. 1987;38:69. [Google Scholar]
- 71.Min D.B., Boff J.M. Chemistry and reaction of singlet oxygen in foods. Compr. Rev. Food Sci. Food Saf. 2002;1:58–72. doi: 10.1111/j.1541-4337.2002.tb00007.x. [DOI] [PubMed] [Google Scholar]
- 72.Devasagayam T., Kamat J.P. 2002. Biological Significance of Singlet Oxygen. [PubMed] [Google Scholar]
- 73.Nikolaidis M.G., Kyparos A., Spanou C., Paschalis V., Theodorou A.A., Vrabas I.S. Redox biology of exercise: an integrative and comparative consideration of some overlooked issues. J. Exp. Biol. 2012;215:1615–1625. doi: 10.1242/jeb.067470. [DOI] [PubMed] [Google Scholar]
- 74.Treml J., Šmejkal K. Flavonoids as potent scavengers of hydroxyl radicals. Compr. Rev. Food Sci. Food Saf. 2016;15:720–738. doi: 10.1111/1541-4337.12204. [DOI] [PubMed] [Google Scholar]
- 75.Waterhouse A.L., Laurie V.F. Oxidation of wine phenolics: a critical evaluation and hypotheses. Am. J. Enol. Vitic. 2006;57:306. [Google Scholar]
- 76.Danilewicz J.C. Review of reaction mechanisms of oxygen and proposed intermediate reduction products in wine: central role of iron and copper. Am. J. Enol. Vitic. 2003;54:73. [Google Scholar]
- 77.Perez-Benito J.F. Iron(III)−Hydrogen peroxide reaction: kinetic evidence of a hydroxyl-mediated chain mechanism. J. Phys. Chem. A. 2004;108:4853–4858. doi: 10.1021/jp031339l. [DOI] [Google Scholar]
- 78.Nguyen T.H., Waterhouse A.L. Acid complexation of iron controls the fate of hydrogen peroxide in model wine. Food Chem. 2022;377 doi: 10.1016/j.foodchem.2021.131910. [DOI] [PubMed] [Google Scholar]
- 79.Dunford H. Oxidations of iron(II)/(III) by hydrogen peroxide: from aquo to enzyme. Coord. Chem. Rev. 2002;233–234:311–318. doi: 10.1016/S0010-8545(02)00024-3. [DOI] [Google Scholar]
- 80.Elias R.J., Waterhouse A.L. Controlling the Fenton reaction in wine. J. Agric. Food Chem. 2010;58:1699–1707. doi: 10.1021/jf903127r. [DOI] [PubMed] [Google Scholar]
- 81.Vangijzegem T., Lecomte V., Ternad I., Van Leuven L., Muller R.N., Stanicki D., Laurent S. Superparamagnetic iron oxide nanoparticles (SPION): from fundamentals to state-of-the-art innovative applications for cancer therapy. Pharmaceutics. 2023;15:236. doi: 10.3390/pharmaceutics15010236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Renner H., Richling E., Durner D. Investigation of molecular changes in wine during storage in refrigerated cabinets. Lebensmittelchemie. 2023;77 doi: 10.1002/lemi.202352227. [DOI] [Google Scholar]
- 83.Jung R., Kumar K., Patz C., Rauhut D., Tarasov A., Schüßler C. Influence of transport temperature profiles on wine quality. Food Packag. Shelf Life. 2021;29 doi: 10.1016/j.fpsl.2021.100706. [DOI] [Google Scholar]
- 84.Ricci A., Parpinello G.P., Versari A. Modelling the evolution of oxidative browning during storage of white wines: effects of packaging and closures. Int. J. Food Sci. Technol. 2017;52:472–479. doi: 10.1111/ijfs.13303. [DOI] [Google Scholar]
- 85.Oliveira C.M., Barros A.S., Silva Ferreira A.C., Silva A.M.S. Influence of the temperature and oxygen exposure in red Port wine: a kinetic approach. Food Res. Int. 2015;75:337–347. doi: 10.1016/j.foodres.2015.06.024. [DOI] [PubMed] [Google Scholar]
- 86.Falconer R.J., Marangon M., Van Sluyter S.C., Neilson K.A., Chan C., Waters E.J. Thermal stability of thaumatin-like protein, chitinase, and invertase isolated from sauvignon blanc and semillon juice and their role in haze formation in wine. J. Agric. Food Chem. 2010;58:975–980. doi: 10.1021/jf902843b. [DOI] [PubMed] [Google Scholar]
- 87.Hopfer H., Buffon P.A., Ebeler S.E., Heymann H. The combined effects of storage temperature and packaging on the sensory, chemical, and physical properties of a cabernet sauvignon wine. J. Agric. Food Chem. 2013;61:3320–3334. doi: 10.1021/jf3051736. [DOI] [PubMed] [Google Scholar]
- 88.Hopfer H., Ebeler S.E., Heymann H. The combined effects of storage temperature and packaging type on the sensory and chemical properties of chardonnay. J. Agric. Food Chem. 2012;60:10743–10754. doi: 10.1021/jf302910f. [DOI] [PubMed] [Google Scholar]
- 89.Giraud E., Ivanova A., Gordon C.S., Whelan J., Considine M.J. Sulphur dioxide evokes a large scale reprogramming of the grape berry transcriptome associated with oxidative signalling and biotic defence responses. Plant Cell Environ. 2012;35:405–417. doi: 10.1111/j.1365-3040.2011.02379.x. [DOI] [PubMed] [Google Scholar]
- 90.Grainger K. 2009. Wine Faults and Flaws; pp. 67–77. Wine Qual. [Google Scholar]
- 91.Petruk G., Del Giudice R., Rigano M.M., Monti D.M. Antioxidants from plants protect against skin photoaging. Oxid. Med. Cell. Longev. 2018;2018:1–11. doi: 10.1155/2018/1454936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Clark A.C., Dias D.A., Smith T.A., Ghiggino K.P., Scollary G.R. Iron(III) tartrate as a potential precursor of light-induced oxidative degradation of white wine: studies in a model wine system. J. Agric. Food Chem. 2011;59:3575–3581. doi: 10.1021/jf104897z. [DOI] [PubMed] [Google Scholar]
- 93.Waterhouse Andrew L., Frost S., Ugliano M., Cantu A.R., Currie B.L., Anderson M., Chassy A.W., Vidal S., Diéval J.-B., Aagaard O., Heymann H. Sulfur dioxide–oxygen consumption ratio reveals differences in bottled wine oxidation. Am. J. Enol. Vitic. 2016;67:449–459. doi: 10.5344/ajev.2016.16006. [DOI] [Google Scholar]
- 94.Waterhouse Andrew L., Sacks G.L., Jeffery D.W. John Wiley & Sons; 2016. Understanding Wine Chemistry. [Google Scholar]
- 95.Grant-Preece P., Barril C., Schmidtke L.M., Scollary G.R., Clark A.C. Light-induced changes in bottled white wine and underlying photochemical mechanisms. Crit. Rev. Food Sci. Nutr. 2017;57:743–754. doi: 10.1080/10408398.2014.919246. [DOI] [PubMed] [Google Scholar]
- 96.Bonamente E., Scrucca F., Rinaldi S., Merico M.C., Asdrubali F., Lamastra L. Environmental impact of an Italian wine bottle: carbon and water footprint assessment. Sci. Total Environ. 2016;560–561:274–283. doi: 10.1016/j.scitotenv.2016.04.026. [DOI] [PubMed] [Google Scholar]
- 97.Caillé S., Samson A., Wirth J., Diéval J.-B., Vidal S., Cheynier V. Sensory characteristics changes of red Grenache wines submitted to different oxygen exposures pre and post bottling. Anal. Chim. Acta. 2010;660:35–42. doi: 10.1016/j.aca.2009.11.049. [DOI] [PubMed] [Google Scholar]
- 98.Revi M., Badeka A., Kontakos S., Kontominas M.G. Effect of packaging material on enological parameters and volatile compounds of dry white wine. Food Chem. 2014;152:331–339. doi: 10.1016/j.foodchem.2013.11.136. [DOI] [PubMed] [Google Scholar]
- 99.Guaita M., Petrozziello M., Motta S., Bonello F., Cravero M.C., Marulli C., Bosso A. Effect of the closure type on the evolution of the physical‐chemical and sensory characteristics of a montepulciano d'Abruzzo rosé wine. J. Food Sci. 2013;78 doi: 10.1111/1750-3841.12022. [DOI] [PubMed] [Google Scholar]
- 100.Karbowiak T., Crouvisier-Urion K., Lagorce A., Ballester J., Geoffroy A., Roullier-Gall C., Chanut J., Gougeon R.D., Schmitt-Kopplin P., Bellat J.-P. Wine aging: a bottleneck story. Npj Sci. Food. 2019;3:14. doi: 10.1038/s41538-019-0045-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vidal J.-C., Caillé S., Samson A., Salmon J.-M. Comparison of the effect of 8 closures in controlled industrial conditions on the shelf life of a red wine. BIO Web Conf. 2017;9 doi: 10.1051/bioconf/20170902024. [DOI] [Google Scholar]
- 102.Strobl M. Red Wine Technology. Elsevier; 2019. Red wine bottling and packaging; pp. 323–339. [DOI] [Google Scholar]
- 103.Gambuti A., Rinaldi A., Ugliano M., Moio L. Evolution of phenolic compounds and astringency during aging of red wine: effect of oxygen exposure before and after bottling. J. Agric. Food Chem. 2013;61:1618–1627. doi: 10.1021/jf302822b. [DOI] [PubMed] [Google Scholar]
- 104.Oliveira V., Lopes P., Cabral M., Pereira H. Kinetics of oxygen ingress into wine bottles closed with natural cork stoppers of different qualities. Am. J. Enol. Vitic. 2013;64:395–399. doi: 10.5344/ajev.2013.13009. [DOI] [Google Scholar]
- 105.Coelho C., Julien P., Nikolantonaki M., Noret L., Magne M., Ballester J., Gougeon R.D. Molecular and macromolecular changes in bottle-aged white wines reflect oxidative evolution–impact of must clarification and bottle closure. Front. Chem. 2018;6:95. doi: 10.3389/fchem.2018.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.He J., Zhou Q., Peck J., Soles R., Qian M.C. The effect of wine closures on volatile sulfur and other compounds during post‐bottle ageing. Flavour Fragr. J. 2013;28:118–128. doi: 10.1002/ffj.3137. [DOI] [Google Scholar]
- 107.Lagorce-Tachon A., Karbowiak T., Paulin C., Simon J.-M., Gougeon R.D., Bellat J.-P. About the role of the bottleneck/cork interface on oxygen transfer. J. Agric. Food Chem. 2016;64:6672–6675. doi: 10.1021/acs.jafc.6b02465. [DOI] [PubMed] [Google Scholar]
- 108.Crouvisier-Urion K., Bellat J.-P., Gougeon R.D., Karbowiak T. Gas transfer through wine closures: a critical review. Trends Food Sci. Technol. 2018;78:255–269. doi: 10.1016/j.tifs.2018.05.021. [DOI] [Google Scholar]
- 109.Fonseca A.L., Brazinha C., Pereira H., Crespo J.G., Teodoro O.M.N.D. Permeability of cork for water and ethanol. J. Agric. Food Chem. 2013;61:9672–9679. doi: 10.1021/jf4015729. [DOI] [PubMed] [Google Scholar]
- 110.Lequin S., Chassagne D., Karbowiak T., Simon J.-M., Paulin C., Bellat J.-P. Diffusion of oxygen in cork. J. Agric. Food Chem. 2012;60:3348–3356. doi: 10.1021/jf204655c. [DOI] [PubMed] [Google Scholar]
- 111.Pons A., Lavigne V., Thibon C., Redon P., Loisel C., Dubourdieu D., Darriet P. Impact of closure OTR on the volatile compound composition and oxidation aroma intensity of sauvignon blanc wines during and after 10 Years of bottle storage. J. Agric. Food Chem. 2021;69:9883–9894. doi: 10.1021/acs.jafc.1c02635. [DOI] [PubMed] [Google Scholar]
- 112.Han G., Ugliano M., Currie B., Vidal S., Diéval J.-B., Waterhouse A.L. Influence of closure, phenolic levels and microoxygenation on Cabernet Sauvignon wine composition after 5 years' bottle storage: influence of closure, phenolic levels and microoxygenation on wine composition. J. Sci. Food Agric. 2015;95:36–43. doi: 10.1002/jsfa.6694. [DOI] [PubMed] [Google Scholar]
- 113.Nevares I., Del Alamo-Sanza M. Characterization of the oxygen transmission rate of new-ancient natural materials for wine maturation containers. Foods. 2021;10:140. doi: 10.3390/foods10010140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chanut J., Bellat J.-P., Gougeon R.D., Karbowiak T. Controlled diffusion by thin layer coating: the intricate case of the glass-stopper interface. Food Control. 2021;120 doi: 10.1016/j.foodcont.2020.107446. [DOI] [Google Scholar]
- 115.Sefton M.A., Simpson R.F. Compounds causing cork taint and the factors affecting their transfer from natural cork closures to wine – a review. Aust. J. Grape Wine Res. 2005;11:226–240. doi: 10.1111/j.1755-0238.2005.tb00290.x. [DOI] [Google Scholar]
- 116.Georgiev V., Ananga A., Tsolova V. Recent advances and uses of grape flavonoids as nutraceuticals. Nutrients. 2014;6:391–415. doi: 10.3390/nu6010391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Snopek L., Mlcek J., Sochorova L., Baron M., Hlavacova I., Jurikova T., Kizek R., Sedlackova E., Sochor J. Contribution of red wine consumption to human health protection. Molecules. 2018;23:1684. doi: 10.3390/molecules23071684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lin Y., Yngve A., Lagergren J., Lu Y. A dietary pattern rich in lignans, quercetin and resveratrol decreases the risk of oesophageal cancer. Br. J. Nutr. 2014;112:2002–2009. doi: 10.1017/S0007114514003055. [DOI] [PubMed] [Google Scholar]
- 119.Oi N., Jeong C.-H., Nadas J., Cho Y.-Y., Pugliese A., Bode A.M., Dong Z. Resveratrol, a red wine polyphenol, suppresses pancreatic cancer by inhibiting leukotriene A4 hydrolase. Cancer Res. 2010;70:9755–9764. doi: 10.1158/0008-5472.CAN-10-2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Caimi G., Carollo C., Presti R.L. Diabetes mellitus: oxidative stress and wine. Curr. Med. Res. Opin. 2003;19:581–586. doi: 10.1185/030079903125002324. [DOI] [PubMed] [Google Scholar]
- 121.Markoski M.M., Garavaglia J., Oliveira A., Olivaes J., Marcadenti A. Molecular properties of red wine compounds and cardiometabolic benefits. Nutr. Metab. Insights. 2016;9 doi: 10.4137/NMI.S32909. NMI.S32909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Di Lorenzo C., Stockley C., Colombo F., Biella S., Orgiu F., Dell'Agli M., Restani P. The role of wine in modulating inflammatory processes: a review. Beverages. 2018;4:88. doi: 10.3390/beverages4040088. [DOI] [Google Scholar]
- 123.Grujic-Milanovic J., Miloradovic Z., Jovovic D., Jacevic V., Milosavljevic I., Milanovic S.D., Mihailovic-Stanojevic N. The red wine polyphenol, resveratrol improves hemodynamics, oxidative defence and aortal structure in essential and malignant hypertension. J. Funct.Foods. 2017;34:266–276. doi: 10.1016/j.jff.2017.04.035. [DOI] [Google Scholar]
- 124.Rodrigo R., Miranda A., Vergara L. Modulation of endogenous antioxidant system by wine polyphenols in human disease. Clin. Chim. Acta. 2011;412:410–424. doi: 10.1016/j.cca.2010.11.034. [DOI] [PubMed] [Google Scholar]
- 125.Tanaka T., Iuchi A., Harada H., Hashimoto S. Potential beneficial effects of wine flavonoids on allergic diseases. Diseases. 2019;7:8. doi: 10.3390/diseases7010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Caruana M., Cauchi R., Vassallo N. Putative role of red wine polyphenols against brain pathology in alzheimer's and Parkinson's disease. Front. Nutr. 2016;3 doi: 10.3389/fnut.2016.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang T., Hammond E.G. Oxidation in Foods and Beverages and Antioxidant Applications. Elsevier; 2010. Lipoxygenase and lipid oxidation in foods; pp. 105–121. [DOI] [Google Scholar]
- 128.Zorraquín-Peña I., Esteban-Fernández A., González De Llano D., Bartolomé B., Moreno-Arribas M.V. Wine-derived phenolic metabolites in the digestive and brain function. Beverages. 2019;5:7. doi: 10.3390/beverages5010007. [DOI] [Google Scholar]
- 129.Giacosa S., Río Segade S., Cagnasso E., Caudana A., Rolle L., Gerbi V. Red Wine Technology. Elsevier; 2019. SO2 in wines; pp. 309–321. [DOI] [Google Scholar]
- 130.Sioumis N., Kallithraka S., Tsoutsouras E., Makris D.P., Kefalas P. Browning development in white wines: dependence on compositional parameters and impact on antioxidant characteristics. Eur. Food Res. Technol. 2005;220:326–330. doi: 10.1007/s00217-004-1032-0. [DOI] [Google Scholar]
- 131.Barbe J.-C., De Revel G., Joyeux A., Lonvaud-Funel A., Bertrand A. Role of carbonyl compounds in SO 2 binding phenomena in musts and wines from botrytized grapes. J. Agric. Food Chem. 2000;48:3413–3419. doi: 10.1021/jf991359d. [DOI] [PubMed] [Google Scholar]
- 132.Guerrero R.F., Cantos-Villar E. Demonstrating the efficiency of sulphur dioxide replacements in wine: a parameter review. Trends Food Sci. Technol. 2015;42:27–43. doi: 10.1016/j.tifs.2014.11.004. [DOI] [Google Scholar]
- 133.Osborne J.P., Dube Morneau A., Mira De Orduna R. Degradation of free and sulfur-dioxide-bound acetaldehyde by malolactic lactic acid bacteria in white wine. J. Appl. Microbiol. 2006;101:474–479. doi: 10.1111/j.1365-2672.2006.02947.x. [DOI] [PubMed] [Google Scholar]
- 134.Wildenradt H.L., Singleton V.L. The production of aldehydes as a result of oxidation of polyphenolic compounds and its relation to wine aging. Am. J. Enol. Vitic. 1974;25:119–126. doi: 10.5344/ajev.1974.25.2.119. [DOI] [Google Scholar]
- 135.Beausoleil J.L., Fiedler J., Spergel J.M. Food intolerance and childhood asthma: what is the link? Pediatr. Drugs. 2007;9:157–163. doi: 10.2165/00148581-200709030-00004. [DOI] [PubMed] [Google Scholar]
- 136.Delfini C., Gaia P., Schellino R., Strano M., Pagliara A., Ambrò S. Fermentability of grape must after inhibition with dimethyl dicarbonate (DMDC) J. Agric. Food Chem. 2002;50:5605–5611. doi: 10.1021/jf0256337. [DOI] [PubMed] [Google Scholar]
- 137.Fredericks I.N., Du Toit M., Krügel M. Efficacy of ultraviolet radiation as an alternative technology to inactivate microorganisms in grape juices and wines. Food Microbiol. 2011;28:510–517. doi: 10.1016/j.fm.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 138.Musfirah M., Rangkuti A.F., Primasari I.A., Nurullita I., Santri B.J., Lana A.T. Environmental health risk analysis of sulfur dioxide (SO2) inhalation exposure in ambient air among the TIRTONIRMOLO community, bantul. J. Environ. Health. 2024;16 [Google Scholar]
- 139.Ough C.S., Were L. Sulfur dioxide and sulfites. FOOD Sci. Technol.-N. Y.-MARCEL DEKKER. 2005;145:143. [Google Scholar]
- 140.Qin G., Meng Z. Effects of sulfur dioxide derivatives on expression of oncogenes and tumor suppressor genes in human bronchial epithelial cells. Food Chem. Toxicol. 2009;47:734–744. doi: 10.1016/j.fct.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 141.Vally H., Misso N.L.A., Madan V. Clinical effects of sulphite additives. Clin. Exp. Allergy. 2009;39:1643–1651. doi: 10.1111/j.1365-2222.2009.03362.x. [DOI] [PubMed] [Google Scholar]
- 142.Vally H., Thompson P. Allergic and asthmatic reactions to alcoholic drinks. Addict. Biol. 2003;8:3–11. doi: 10.1080/1355621031000069828. [DOI] [PubMed] [Google Scholar]
- 143.Wüthrich B. Allergic and intolerance reactions to wine. Allergol. Sel. 2018;2:80. doi: 10.5414/ALX01420E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wigand P., Blettner M., Saloga J., Decker H. Prevalence of wine intolerance: results of a survey from Mainz, Germany. Dtsch. Ärztebl. Int. 2012;109:437. doi: 10.3238/arztebl.2012.0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ribéreau-Gayon P., Glories Y., Maujean A., Dubourdieu D., Darriet P., Towey J. 2006. The Chemistry of Wine Stabilization and Treatments. No Title. [Google Scholar]
- 146.Garde-Cerdán T., Marsellés-Fontanet A.R., Arias-Gil M., Ancín-Azpilicueta C., Martín-Belloso O. Influence of SO2 on the evolution of volatile compounds through alcoholic fermentation of must stabilized by pulsed electric fields. Eur. Food Res. Technol. 2008;227:401–408. doi: 10.1007/s00217-007-0734-5. [DOI] [Google Scholar]
- 147.Costello P.J., Henschke P.A. Mousy off-flavor of wine: precursors and biosynthesis of the causative N-heterocycles 2-ethyltetrahydropyridine, 2-acetyltetrahydropyridine, and 2-Acetyl-1-pyrroline by Lactobacillus hilgardii DSM 20176. J. Agric. Food Chem. 2002;50:7079–7087. doi: 10.1021/jf020341r. [DOI] [PubMed] [Google Scholar]
- 148.Landete J.M., Ferrer S., Pardo I. Biogenic amine production by lactic acid bacteria, acetic bacteria and yeast isolated from wine. Food Control. 2007;18:1569–1574. doi: 10.1016/j.foodcont.2006.12.008. [DOI] [Google Scholar]
- 149.Ough C.S., Crowell E.A. Use of sulfur dioxide in winemaking. J. Food Sci. 1987;52:386–388. doi: 10.1111/j.1365-2621.1987.tb06620.x. [DOI] [Google Scholar]
- 150.Puértolas E., López N., Condón S., Raso J., Álvarez I. Pulsed electric fields inactivation of wine spoilage yeast and bacteria. Int. J. Food Microbiol. 2009;130:49–55. doi: 10.1016/j.ijfoodmicro.2008.12.035. [DOI] [PubMed] [Google Scholar]
- 151.Santos M.C., Nunes C., Saraiva J.A., Coimbra M.A. Chemical and physical methodologies for the replacement/reduction of sulfur dioxide use during winemaking: review of their potentialities and limitations. Eur. Food Res. Technol. 2012;234:1–12. doi: 10.1007/s00217-011-1614-6. [DOI] [Google Scholar]
- 152.Suárez R., Suárez-Lepe J.A., Morata A., Calderón F. The production of ethylphenols in wine by yeasts of the genera Brettanomyces and Dekkera: a review. Food Chem. 2007;102:10–21. doi: 10.1016/j.foodchem.2006.03.030. [DOI] [Google Scholar]
- 153.Garde-Cerdán T., Marsellés-Fontanet A.R., Arias-Gil M., Martín-Belloso O., Ancín-Azpilicueta C. Influence of SO2 on the consumption of nitrogen compounds through alcoholic fermentation of must sterilized by pulsed electric fields. Food Chem. 2007;103:771–777. doi: 10.1016/j.foodchem.2006.09.018. [DOI] [Google Scholar]
- 154.Solieri L., Giudici P. Development of a sequence-characterized amplified region marker-targeted quantitative PCR assay for strain-specific detection of Oenococcus oeni during wine malolactic fermentation. Appl. Environ. Microbiol. 2010;76:7765–7774. doi: 10.1128/AEM.00929-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pérez-Magariño S., Cano-Mozo E., Albors C., Santos A., Navascués E. Autochthonous Oenococcus oeni strain to avoid histamine formation in red wines: a study in real winemaking conditions. Am. J. Enol. Vitic. 2021;72:170–180. doi: 10.5344/ajev.2020.20010. [DOI] [Google Scholar]
- 156.Azzolini M., Tosi E., Veneri G., Zapparoli G. Evaluating the efficacy of lysozyme against lactic acid bacteria under different winemaking scenarios. South Afr. J. Enol. Vitic. 2010;31:99–105. [Google Scholar]
- 157.Liburdi K., Benucci I., Esti M. Lysozyme in wine: an overview of current and future applications. Compr. Rev. Food Sci. Food Saf. 2014;13:1062–1073. doi: 10.1111/1541-4337.12102. [DOI] [Google Scholar]
- 158.Delfini C., Cersosimo M., Del Prete V., Strano M., Gaetano G., Pagliara A., Ambrò S. Resistance screening essay of wine lactic acid bacteria on lysozyme: efficacy of lysozyme in unclarified grape musts. J. Agric. Food Chem. 2004;52:1861–1866. doi: 10.1021/jf034824m. [DOI] [PubMed] [Google Scholar]
- 159.Guzzo F., Cappello M.S., Azzolini M., Tosi E., Zapparoli G. The inhibitory effects of wine phenolics on lysozyme activity against lactic acid bacteria. Int. J. Food Microbiol. 2011 doi: 10.1016/j.ijfoodmicro.2011.05.023. S0168160511003187. [DOI] [PubMed] [Google Scholar]
- 160.Bustamante M., Giménez P., Just-Borràs A., Solé-Clua I., Gombau J., Heras J.M., Sieczkowski N., Gil M., Pérez-Navarro J., Gómez-Alonso S., Canals J.M., Zamora F. Use of glutathione, pure or as a specific inactivated yeast, as an alternative to sulphur dioxide for protecting white grape must from browning. Foods. 2024;13:310. doi: 10.3390/foods13020310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gambuti Angelita, Picariello L., Rolle L., Moio L. Evaluation of the use of sulfur dioxide and glutathione to prevent oxidative degradation of malvidin-3-monoglucoside by hydrogen peroxide in the model solution and real wine. Food Res. Int. 2017;99:454–460. doi: 10.1016/j.foodres.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 162.Comuzzo P., Battistutta F., Vendrame M., Páez M.S., Luisi G., Zironi R. Antioxidant properties of different products and additives in white wine. Food Chem. 2015;168:107–114. doi: 10.1016/j.foodchem.2014.07.028. [DOI] [PubMed] [Google Scholar]
- 163.Divol B., Strehaiano P., Lonvaud-Funel A. Effectiveness of dimethyldicarbonate to stop alcoholic fermentation in wine. Food Microbiol. 2005;22:169–178. doi: 10.1016/j.fm.2004.07.003. [DOI] [Google Scholar]
- 164.Bartowsky E.J. Bacterial spoilage of wine and approaches to minimize it. Lett. Appl. Microbiol. 2009;48:149–156. doi: 10.1111/j.1472-765X.2008.02505.x. [DOI] [PubMed] [Google Scholar]
- 165.Chung W., Hancock R.E.W. Action of lysozyme and nisin mixtures against lactic acid bacteria. Int. J. Food Microbiol. 2000;60:25–32. doi: 10.1016/S0168-1605(00)00330-5. [DOI] [PubMed] [Google Scholar]
- 166.Rojo-Bezares B., Sáenz Y., Zarazaga M., Torres C., Ruiz-Larrea F. Antimicrobial activity of nisin against Oenococcus oeni and other wine bacteria. Int. J. Food Microbiol. 2007;116:32–36. doi: 10.1016/j.ijfoodmicro.2006.12.020. [DOI] [PubMed] [Google Scholar]
- 167.Kjos M., Borrero J., Opsata M., Birri D.J., Holo H., Cintas L.M., Snipen L., Hernández P.E., Nes I.F., Diep D.B. Target recognition, resistance, immunity and genome mining of class II bacteriocins from Gram-positive bacteria. Microbiology. 2011;157:3256–3267. doi: 10.1099/mic.0.052571-0. [DOI] [PubMed] [Google Scholar]
- 168.Egan K., Field D., Rea M.C., Ross R.P., Hill C., Cotter P.D. Bacteriocins: novel solutions to age old spore-related problems? Front. Microbiol. 2016;7 doi: 10.3389/fmicb.2016.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Pérez-Serradilla J.A., De Castro M.D.L. Role of lees in wine production: a review. Food Chem. 2008;111:447–456. doi: 10.1016/j.foodchem.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 170.Fornairon-Bonnefond C., Salmon J.-M. Impact of oxygen consumption by yeast lees on the autolysis phenomenon during simulation of wine aging on lees. J. Agric. Food Chem. 2003;51:2584–2590. doi: 10.1021/jf0259819. [DOI] [PubMed] [Google Scholar]
- 171.Gallardo-Chacón J.J., Vichi S., Urpí P., López-Tamames E., Buxaderas S. Antioxidant activity of lees cell surface during sparkling wine sur lie aging. Int. J. Food Microbiol. 2010;143:48–53. doi: 10.1016/j.ijfoodmicro.2010.07.027. [DOI] [PubMed] [Google Scholar]
- 172.Jaehrig S.C., Rohn S., Kroh L.W., Fleischer L.-G., Kurz T. In vitro potential antioxidant activity of (1→3),(1→6)-β- d -glucan and protein fractions from Saccharomyces cerevisiae cell walls. J. Agric. Food Chem. 2007;55:4710–4716. doi: 10.1021/jf063209q. [DOI] [PubMed] [Google Scholar]
- 173.Silver S., Phung L.T., Silver G. Silver as biocides in burn and wound dressings and bacterial resistance to silver compounds. J. Ind. Microbiol. Biotechnol. 2006;33:627–634. doi: 10.1007/s10295-006-0139-7. [DOI] [PubMed] [Google Scholar]
- 174.Klasen H.J. A historical review of the use of silver in the treatment of burns. II. Renewed interest for silver. Burns. 2000;26:131–138. doi: 10.1016/S0305-4179(99)00116-3. [DOI] [PubMed] [Google Scholar]
- 175.Izquierdo-Cañas P.M., García-Romero E., Huertas-Nebreda B., Gómez-Alonso S. Colloidal silver complex as an alternative to sulphur dioxide in winemaking. Food Control. 2012;23:73–81. doi: 10.1016/j.foodcont.2011.06.014. [DOI] [Google Scholar]
- 176.Marambio-Jones C., Hoek E.M.V. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J. Nanoparticle Res. 2010;12:1531–1551. doi: 10.1007/s11051-010-9900-y. [DOI] [Google Scholar]
- 177.Pradeep T., Anshup Noble metal nanoparticles for water purification: a critical review. Thin Solid Films. 2009;517:6441–6478. doi: 10.1016/j.tsf.2009.03.195. [DOI] [Google Scholar]
- 178.Emamifar A., Kadivar M., Shahedi M., Soleimanian-Zad S. Effect of nanocomposite packaging containing Ag and ZnO on inactivation of Lactobacillus plantarum in orange juice. Food Control. 2011;22:408–413. doi: 10.1016/j.foodcont.2010.09.011. [DOI] [Google Scholar]
- 179.Barbosa-Cánovas G., Fernández-Molina J., Swanson B. Pulsed electric fields: a novel technology for food preservation. Agro Food Ind. Hi-Tech. 2001;12:9–14. [Google Scholar]
- 180.Buckow R., Schroeder S., Berres P., Baumann P., Knoerzer K. Simulation and evaluation of pilot-scale pulsed electric field (PEF) processing. J. Food Eng. 2010;101:67–77. doi: 10.1016/j.jfoodeng.2010.06.010. [DOI] [Google Scholar]
- 181.Puértolas E., López N., Condón S., Raso J., Álvarez I. Pulsed electric fields inactivation of wine spoilage yeast and bacteria. Int. J. Food Microbiol. 2009;130:49–55. doi: 10.1016/j.ijfoodmicro.2008.12.035. [DOI] [PubMed] [Google Scholar]
- 182.Zhang Q., Barbosa-Cánovas G.V., Swanson B.G. Engineering aspects of pulsed electric field pasteurization. J. Food Eng. 1995;25:261–281. doi: 10.1016/0260-8774(94)00030-D. [DOI] [Google Scholar]
- 183.Butz P., Tauscher B. Emerging technologies: chemical aspects. Food Res. Int. 2002;35:279–284. doi: 10.1016/S0963-9969(01)00197-1. [DOI] [Google Scholar]
- 184.Falguera V., Pagán J., Ibarz A. Effect of UV irradiation on enzymatic activities and physicochemical properties of apple juices from different varieties. LWT - Food Sci. Technol. 2011;44:115–119. doi: 10.1016/j.lwt.2010.05.028. [DOI] [Google Scholar]
- 185.Guerrero-Beltrén J.A., Barbosa-Cénovas G.V. Inactivation of Saccharomyces cerevisiae and polyphenoloxidase in mango nectar treated with UV light. J. Food Protect. 2006;69:362–368. doi: 10.4315/0362-028X-69.2.362. [DOI] [PubMed] [Google Scholar]
- 186.Guerrero R.F., Puertas B., Fernández M.I., Palma M., Cantos-Villar E. Induction of stilbenes in grapes by UV-C: comparison of different subspecies of Vitis. Innov. Food Sci. Emerg. Technol. 2010;11:231–238. doi: 10.1016/j.ifset.2009.10.005. [DOI] [Google Scholar]
- 187.Das M., Das D.K. Resveratrol and cardiovascular health. Mol. Aspects Med. 2010;31:503–512. doi: 10.1016/j.mam.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 188.Upadhyay S., Gupta K.B., Kaur S., Rubal, Kumar S., Mantha A.K., Dhiman M. Resveratrol: a miracle drug for vascular pathologies. Funct. Food Hum. Health. 2018:119–142. [Google Scholar]
- 189.Cao X., Zhang Y., Zhang F., Wang Y., Yi J., Liao X. Effects of high hydrostatic pressure on enzymes, phenolic compounds, anthocyanins, polymeric color and color of strawberry pulps: effects of high hydrostatic pressure on strawberry pulps. J. Sci. Food Agric. 2011;91:877–885. doi: 10.1002/jsfa.4260. [DOI] [PubMed] [Google Scholar]
- 190.Ramirez R., Saraiva J., Pérez Lamela C., Torres J.A. Reaction kinetics analysis of chemical changes in pressure-assisted thermal processing. Food Eng. Rev. 2009;1:16–30. doi: 10.1007/s12393-009-9002-8. [DOI] [Google Scholar]
- 191.Salvador Â.C., Santos M. da C., Saraiva J.A. Effect of the ionic liquid [bmim] Cl and high pressure on the activity of cellulase. Green Chem. 2010;12:632–635. [Google Scholar]
- 192.Ferreira E.H.D.R., Rosenthal A., Calado V., Saraiva J., Mendo S. Byssochlamys nivea inactivation in pineapple juice and nectar using high pressure cycles. J. Food Eng. 2009;95:664–669. doi: 10.1016/j.jfoodeng.2009.06.053. [DOI] [Google Scholar]
- 193.Smelt J.P.P.M. Recent advances in the microbiology of high pressure processing. Trends Food Sci. Technol. 1998;9:152–158. doi: 10.1016/S0924-2244(98)00030-2. [DOI] [Google Scholar]
- 194.Castro S.M., Saraiva J.A., Domingues F.M.J., Delgadillo I. Effect of mild pressure treatments and thermal blanching on yellow bell peppers (Capsicum annuum L.) LWT - Food Sci. Technol. 2011;44:363–369. doi: 10.1016/j.lwt.2010.09.020. [DOI] [Google Scholar]
- 195.Rastogi N.K., Raghavarao K.S.M.S., Balasubramaniam V.M., Niranjan K., Knorr D. Opportunities and challenges in high pressure processing of foods. Crit. Rev. Food Sci. Nutr. 2007;47:69–112. doi: 10.1080/10408390600626420. [DOI] [PubMed] [Google Scholar]
- 196.Correia I., Nunes A., Saraiva J.A., Barros A.S., Delgadillo I. High pressure treatments largely avoid/revert decrease of cooked sorghum protein digestibility when applied before/after cooking. LWT - Food Sci. Technol. 2011;44:1245–1249. doi: 10.1016/j.lwt.2010.10.021. [DOI] [Google Scholar]
- 197.Moerman F. High hydrostatic pressure inactivation of vegetative microorganisms, aerobic and anaerobic spores in pork Marengo, a low acidic particulate food product. Meat Sci. 2005;69:225–232. doi: 10.1016/j.meatsci.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 198.Wuytack E.Y., Diels A.M.J., Michiels C.W. Bacterial inactivation by high-pressure homogenisation and high hydrostatic pressure. Int. J. Food Microbiol. 2002;77:205–212. doi: 10.1016/S0168-1605(02)00054-5. [DOI] [PubMed] [Google Scholar]
- 199.Christofi S., Katsaros G., Mallouchos A., Cotea V., Kallithraka S. Reducing SO2 content in wine by combining High Pressure and glutathione addition. OENO One. 2021;55:235–252. doi: 10.20870/oeno-one.2021.55.1.4558. [DOI] [Google Scholar]
- 200.Daoudi L., Quevedo J.M., Trujillo A.J., Capdevila F., Bartra E., Mínguez S., Guamis B. Effects of high-pressure treatment on the sensory quality of white grape juice. High Press. Res. 2002;22:705–709. doi: 10.1080/08957950212430. [DOI] [Google Scholar]
- 201.Talcott S.T., Brenes C.H., Pires D.M., Del Pozo-Insfran D. Phytochemical stability and color retention of copigmented and processed muscadine grape juice. J. Agric. Food Chem. 2003;51:957–963. doi: 10.1021/jf0209746. [DOI] [PubMed] [Google Scholar]
- 202.Delfini C., Conterno L., Carpi G., Rovere P., Tabusso A., Cocito C., Amati A. Microbiological stabilisation of grape musts and wines by high hydrostatic pressures. J. Wine Res. 1995;6:143–151. doi: 10.1080/09571269508718031. [DOI] [Google Scholar]
- 203.Mok C., Song K., Park Y., Lim S., Ruan R., Chen P. High hydrostatic pressure pasteurization of red wine. J. Food Sci. 2006;71 doi: 10.1111/j.1750-3841.2006.00145.x. [DOI] [Google Scholar]
- 204.Puig A., Vilavella M., Daoudi L., Guamis B., Minguez S. 2003. Microbiological and Biochemical Stabilization of Wines Using the High Pressure Technique. [Google Scholar]
- 205.Christofi S., Malliaris D., Katsaros G., Panagou E., Kallithraka S. Limit SO2 content of wines by applying high hydrostatic pressure. Innov. Food Sci. Emerg. Technol. 2020;62 doi: 10.1016/j.ifset.2020.102342. [DOI] [Google Scholar]
- 206.Thompson-Witrick K.A., Pitts E.R., Nemenyi J.L., Budner D. The impact packaging type has on the flavor of wine. Beverages. 2021;7:36. doi: 10.3390/beverages7020036. [DOI] [Google Scholar]
- 207.Waterhouse A.L., Sacks G.L., Jeffery D.W. John Wiley & Sons; 2024. Understanding Wine Chemistry. [Google Scholar]
- 208.Röcker B., Rüegg N., Glöss A.N., Yeretzian C., Yildirim S. Inactivation of palladium‐based oxygen scavenger system by volatile sulfur compounds present in the headspace of packaged food. Packag. Technol. Sci. 2017;30:427–442. doi: 10.1002/pts.2220. [DOI] [Google Scholar]
- 209.de Gaulejac, Vivas N., Nonier M.-F., Absalon C., Bourgeois G. Study and quantification of monomeric flavan-3-ol and dimeric procyanidin quinonic forms by HPLC/ESI-MS. Application to red wine oxidation. J. Sci. Food Agric. 2001;81:1172–1179. doi: 10.1002/jsfa.926. [DOI] [Google Scholar]