Figure 6.
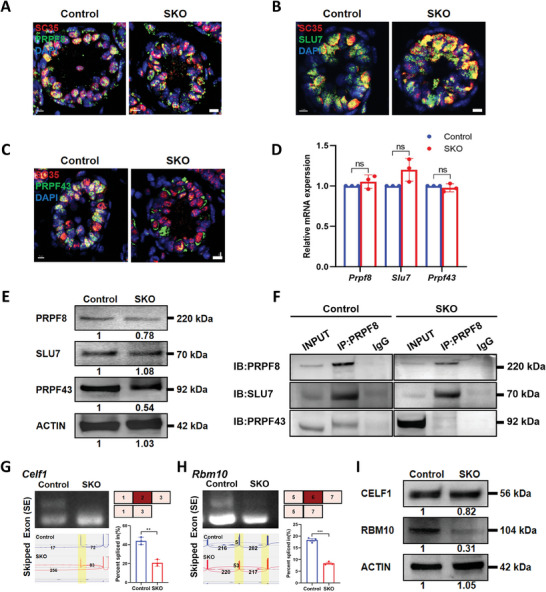
Deletion of CWF19L2 negatively affects spliceosome assembly and stability. A) Co‐immunofluorescence staining of SC35 (red) with the spliceosome skeleton marker PRPF8 (green) in testis sections of PD10 Cwf19l2‐SKO and control mice. DNA was stained with DAPI. Scale bars = 5 µm. B) Co‐immunofluorescence staining of SC35 (red) with the P complex marker SLU7 (green) in testis sections of PD10 Cwf19l2‐SKO and control mice. DNA was stained with DAPI. Scale bars = 5 µm. C) Co‐immunofluorescence staining of SC35 (red) with the ILS complex marker PRPF43 (green) in testis sections of PD10 Cwf19l2‐SKO and control mice. DNA was stained with DAPI. Scale bars = 5 µm. D) QPCR analysis of the mRNA levels of spliceosome‐related genes Prpf8, Slu7, and Prpf43 in sorted germ cells from Cwf19l2‐SKO and control mice. Data are presented as mean ± SD, n = 3, ns: not significant by two‐tailed Student’ s t‐test. E) Immunoblotting analysis of PRPF8, SLU7, and PRPF43 protein in testes of PD10 Cwf19l2‐SKO and control mice. ACTIN served as the loading control. F) Co‐IP assays of the interaction between PRPF8, SLU7, and PRPF43 in sorted germ cells from Cwf19l2‐SKO and control mice. IgG was used as the negative control. G) Visualization and validation of Celf1 abnormal ASEs in sorted germ cells from Cwf19l2‐SKO and control mice. H) Visualization and validation of Rbm10 abnormal ASEs in sorted germ cells from Cwf19l2‐SKO and control mice. I) Immunoblotting analysis of CELF1 and RBM10 protein in testes of PD10 Cwf19l2‐SKO and control mice. ACTIN served as the loading control.
