Abstract
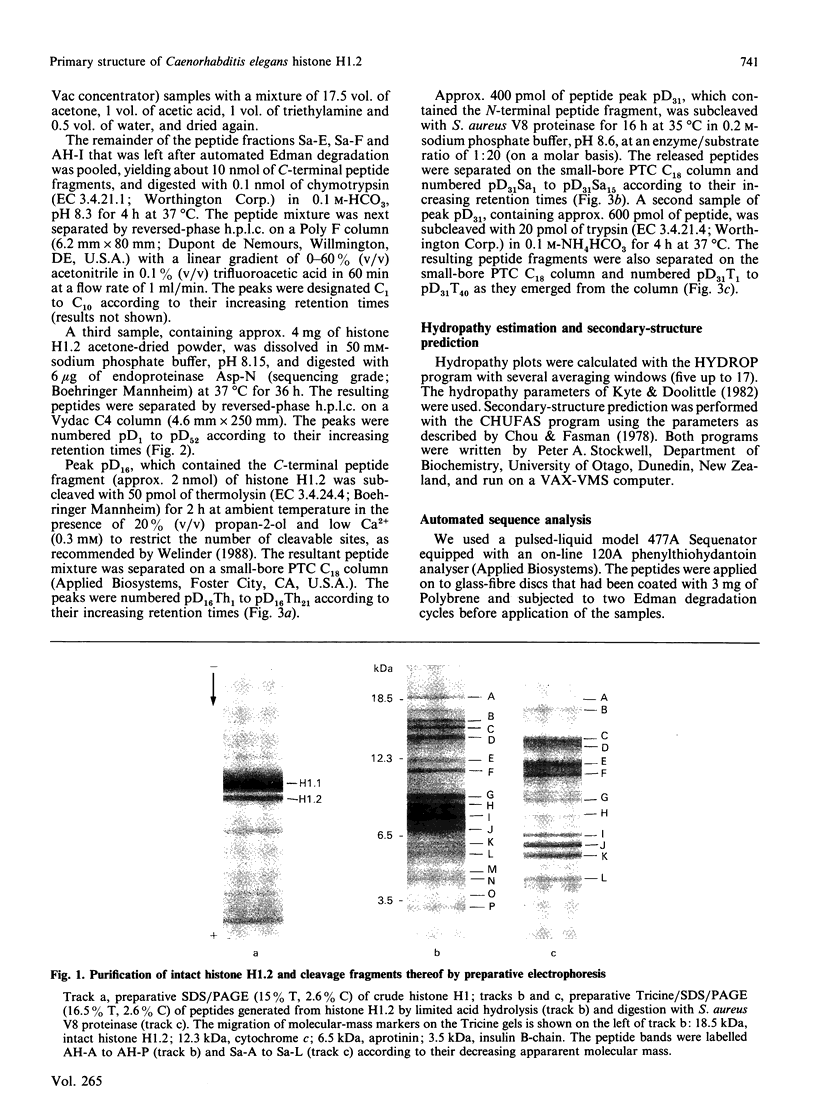
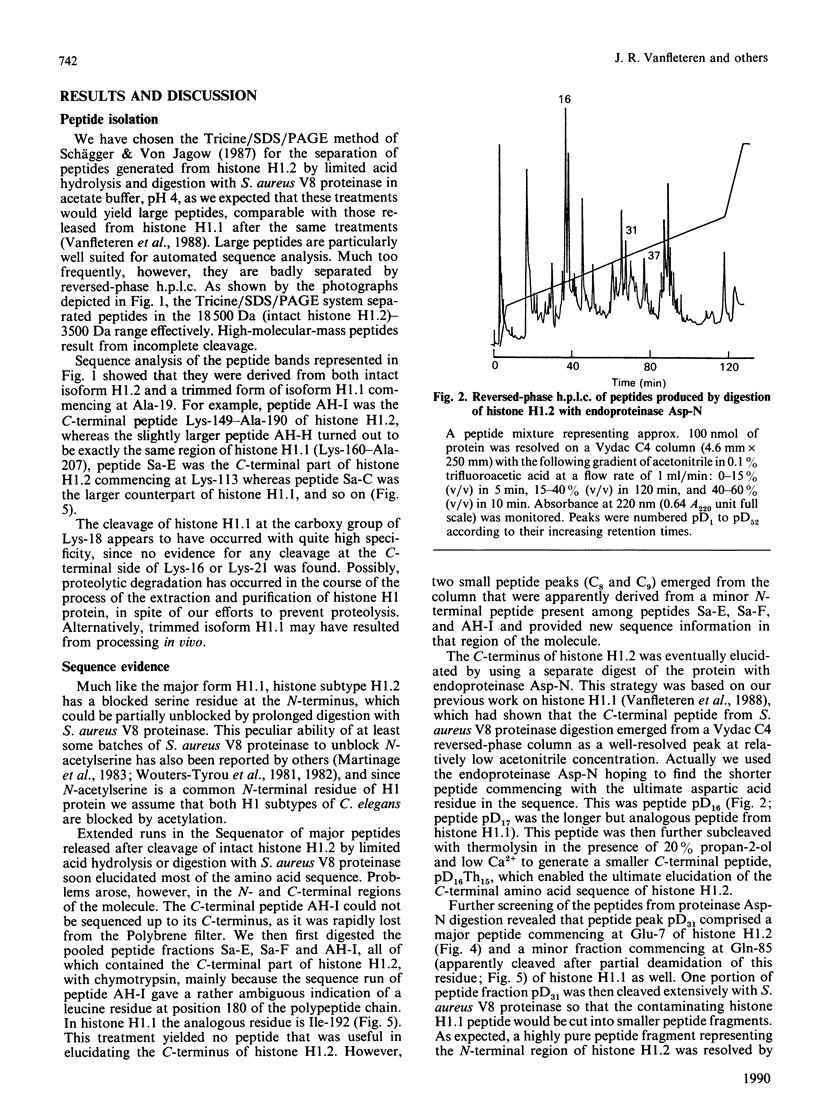
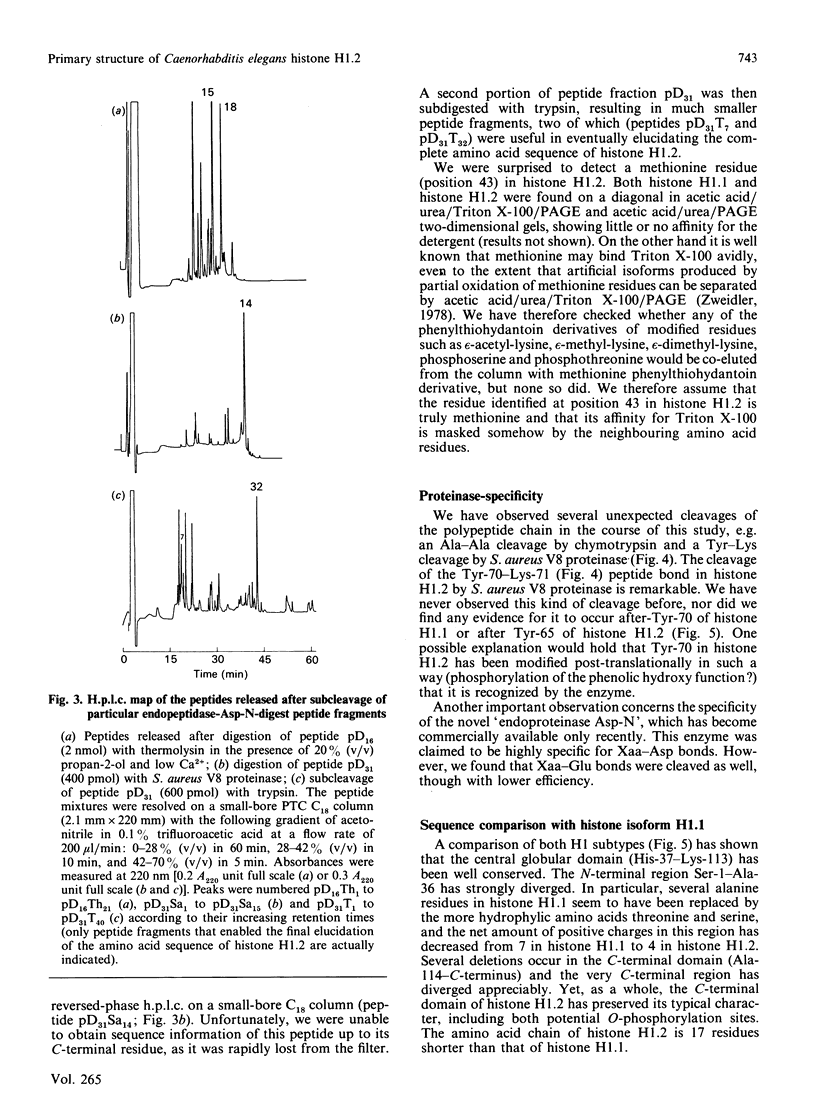
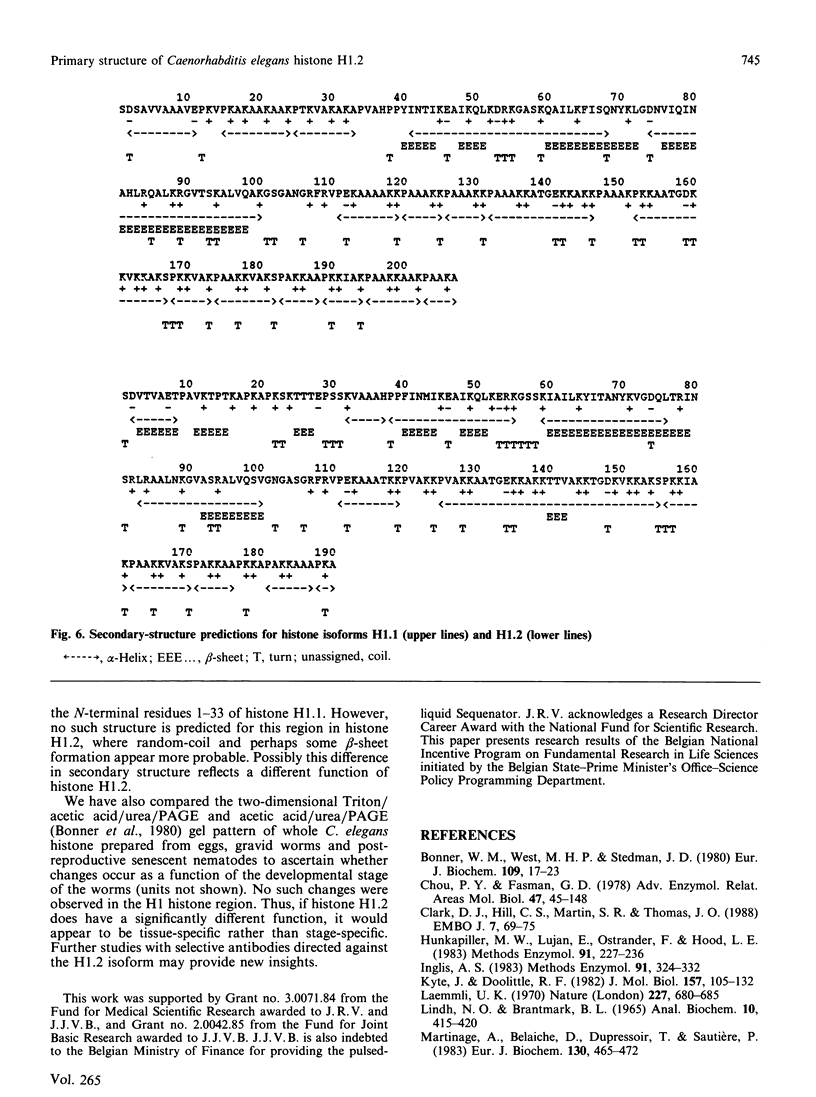
The complete amino acid sequence of a minor isoform (H1.2) of histone H1 from the nematode Caenorhabditis elegans was determined. The amino acid chain consists of 190 residues and has a blocked N-terminus. Histone subtype H1.2 is 17 residues shorter than the major isoform H1.1, mainly as the result of deletions of short peptide fragments. Considerable divergence from isoform H1.1 has occurred in the N-terminal domain and the very C-terminus of the molecule, but the central globular domain and most of the C-terminal domain, including two potential phosphorylation sites, have been well conserved. Secondary-structure predictions for both H1 isoforms reveal a high potential for helix formation in the N-terminal region 1-33 of isoform H1.1 whereas the corresponding region in isoform H1.2 has low probability of being found in alpha-helix. No major differences in secondary structure are predicted for other parts of both H1 subtypes. The aberrant conformation of isoform H1.2 may be indicative of a significantly different function.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., West M. H., Stedman J. D. Two-dimensional gel analysis of histones in acid extracts of nuclei, cells, and tissues. Eur J Biochem. 1980 Aug;109(1):17–23. doi: 10.1111/j.1432-1033.1980.tb04762.x. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Clark D. J., Hill C. S., Martin S. R., Thomas J. O. Alpha-helix in the carboxy-terminal domains of histones H1 and H5. EMBO J. 1988 Jan;7(1):69–75. doi: 10.1002/j.1460-2075.1988.tb02784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunkapiller M. W., Lujan E., Ostrander F., Hood L. E. Isolation of microgram quantities of proteins from polyacrylamide gels for amino acid sequence analysis. Methods Enzymol. 1983;91:227–236. doi: 10.1016/s0076-6879(83)91019-4. [DOI] [PubMed] [Google Scholar]
- Inglis A. S. Cleavage at aspartic acid. Methods Enzymol. 1983;91:324–332. doi: 10.1016/s0076-6879(83)91030-3. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Martinage A., Belaiche D., Dupressoir T., Sautiere P. Primary structure of histone H2A from gonads of the starfish Asterias rubens. Eur J Biochem. 1983 Feb 15;130(3):465–472. doi: 10.1111/j.1432-1033.1983.tb07173.x. [DOI] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Vanfleteren J. R., Van Beeumen J. J. Nematode chromosomal proteins--III. Some structural properties of the histones of Caenorhabditis elegans. Comp Biochem Physiol B. 1983;76(1):179–184. doi: 10.1016/0305-0491(83)90191-8. [DOI] [PubMed] [Google Scholar]
- Vanfleteren J. R., Van Bun S. M., Van Beeumen J. J. The primary structure of the major isoform (H1.1) of histone H1 from the nematode Caenorhabditis elegans. Biochem J. 1988 Oct 15;255(2):647–652. [PMC free article] [PubMed] [Google Scholar]
- Welinder K. G. Generation of peptides suitable for sequence analysis by proteolytic cleavage in reversed-phase high-performance liquid chromatography solvents. Anal Biochem. 1988 Oct;174(1):54–64. doi: 10.1016/0003-2697(88)90518-0. [DOI] [PubMed] [Google Scholar]
- Wouters-Tyrou D., Martin-Ponthieu A., Briand G., Sautiere P., Biserte G. The amino-acid sequence of histone H2A from cuttlefish Sepia officinalis. Eur J Biochem. 1982 Jun;124(3):489–498. doi: 10.1111/j.1432-1033.1982.tb06620.x. [DOI] [PubMed] [Google Scholar]
- Wouters-Tyrou D., Martin-Ponthieu A., Sautiere P., Biserte G. Acetylation of histone H4 in chicken erythrocyte and cuttle-fish testis chromatin. FEBS Lett. 1981 Jun 15;128(2):195–200. doi: 10.1016/0014-5793(81)80079-8. [DOI] [PubMed] [Google Scholar]
- Zweidler A. Resolution of histones by polyacrylamide gel electrophoresis in presence of nonionic detergents. Methods Cell Biol. 1978;17:223–233. [PubMed] [Google Scholar]



