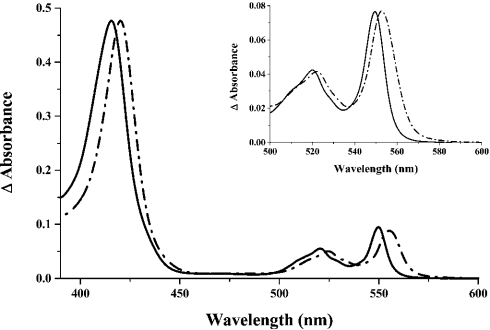
Figure 1. Absorption spectra of the recombinant CXXCH variant of Trypanosoma brucei cytochrome c and of wild-type Crithidia fasciculata cytochrome c.
The CXXCH variant of T. brucei cytochrome c was purified from periplasmic extracts of E. coli cells co-transformed with the plasmid for the E. coli Ccm system. The spectrum (solid line) was recorded at 25 °C with the protein in 50 mM Tris/HCl, pH 7.5, following addition of a few grains of disodium dithionite. Wild-type C. fasciculata cytochrome c was purified from the mitochondria of a chanomastigote culture; the spectrum (broken line) was recorded under the same conditions as above, except at pH 8.0. In the absence of accurate molar absorption coefficients, these spectra have been normalized by the Soret band intensity. Inset: reduced pyridine haemochrome spectra of the CXXCH variant of T. brucei cytochrome c purified from the periplasm of E. coli (solid line) and of C. fasciculata cytochrome c (broken line). The final concentrations of NaOH and pyridine were 0.2 M and 30% (v/v) respectively; spectra were normalized by the α-band intensity.