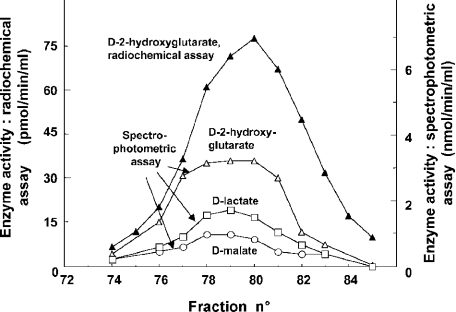
Figure 2. Co-elution of the enzyme utilizing radiolabelled D-2-hydroxyglutarate with a dehydrogenase acting on D-2-hydroxyglutarate, D-malate and D-lactate.
A portion of the elution profile of a DEAE-Sepharose column is shown similar to the one shown in Figure 1. The activity was determined by the radiochemical assay in the presence of 2 μM unlabelled D-2-hydroxyglutarate (▴) or through the oxidation of DCIP in the presence of 1 mM D-2-hydroxyglutarate (▵), D-lactate (□) or D-malate (○). The approx. 50-fold difference in activity between the two conditions is due to a combination of several factors such as the use of subsaturating concentrations of substrate in the radiochemical assay, a primary isotopic effect and the stimulation exerted by electron acceptors (see the Discussion section).