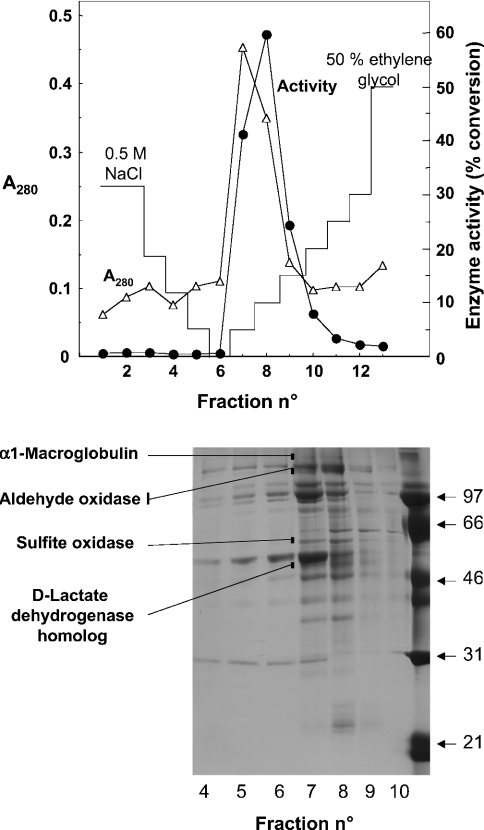
Figure 5. Purification by chromatography on phenyl-Sepharose.
Upper panel: a preparation (9 mg of protein) purified by chromatography on DEAE-Sepharose and Blue Trisacryl was applied on to a phenyl-Sepharose column and eluted with a decreasing gradient of NaCl and an increasing gradient of ethylene glycol. Fractions of 30 ml (fraction 1) and 2 ml (fractions 2–13) were collected. D-2-Hydroxyglutarate dehydrogenase (•) was measured by the radiochemical assay on 10 μl of each fraction. Lower panel: SDS/PAGE of the fractions. The indicated bands were found to co-elute with the activity both in the Blue Trisacryl (results not shown) and the phenyl-Sepharose columns. They were cut from the gel, digested with trypsin and their identity was determined by electrospray ionization–tandem MS.