Abstract
The process of programmed cell death (or apoptosis) occurs widely in tissue maintenance and embryonic development, and is under tight regulatory control. It is now clear that one of the important regulators of apoptosis are G-protein-coupled receptors. In the present study, we investigate the regulatory mechanism employed by the Gq/11-coupled M3-muscarinic receptor in mediating an anti-apoptotic response. Using a CHO (Chinese-hamster ovary) cell model, we demonstrate that the M3-muscarinic receptor anti-apoptotic response is independent of calcium/phospholipase C signalling. This response can, however, be inhibited by the transcriptional inhibitor actinomycin D at a concentration that inhibits the rapid increase in gene transcription mediated by M3-muscarinic receptor stimulation. Furthermore, apoptosis in CHO cells induced by the DNA-damaging agent, etoposide, is associated with a fall in the levels of the anti-apoptotic Bcl-2 protein. This fall in Bcl-2 protein concentration can be attenuated by M3-muscarinic receptor stimulation. We conclude, therefore, that the M3-muscarinic receptor signals to the anti-apoptotic pathway via a mechanism that is independent of calcium/phospholipase C signalling, but in a manner that involves both gene transcription and the up-regulation of Bcl-2 protein.
Keywords: apoptosis, Bcl-2, caspase, G-protein-coupled receptor (GPCR), M3-muscarinic receptor, transcription
Abbreviations: CHO, Chinese-hamster ovary; DEVD-pNA, Asp-Glu-Val-Asp-p-nitroanilide; DTT, dithiothreitol; ERK, extracellular-signal-regulated protein kinase-2; FCS, foetal calf serum; GPCR, G-protein-coupled receptor; KHB, Krebs/Hepes buffer; α-MEM, α-minimal essential medium; PKB, protein kinase B; PLC, phospholipase C; RT, reverse transcriptase; T-TBS, Tween/Tris-buffered saline
INTRODUCTION
GPCRs (G-protein-coupled receptors) represent one of the largest gene families in the mammalian genome responding to ligands as diverse as biogenic amines, peptides, ions and light. The key role that GPCRs play in controlling cellular function rests not only in this diversity of receptor subtypes, but also in the fact that each receptor has the ability to activate a large array of interconnecting, intracellular signalling pathways [1]. The repertoire of signalling pathways controlled by GPCRs has recently been extended by studies linking GPCRs to the regulation of programmed cell death or apoptosis. Depending on the receptor subtype and the cell type in which the receptor is expressed, GPCRs can either induce apoptosis or protect cells from apoptotic stimuli. For example, β1-adrenergic receptors in cardiac myocytes can induce apoptosis through a Gs-mediated pathway, whereas β2-adrenergic receptors in the same cell type can protect cells through a Gi-mediated mechanism [2]. Furthermore, experimental protocols that prevent desensitization of certain GPCRs, such as rhodopsin in the rod outer segment membranes [3,4] and metabotropic glutamate receptors in transfected cell lines [5], result in unregulated GPCR signalling and stimulus-dependent apoptosis.
Prominent among the GPCRs that protect cells from apoptotic stimuli are the subtypes of the muscarinic receptor family [6,7]. Our earlier studies have demonstrated that the muscarinic receptor family members that are coupled to Gq/11-proteins (e.g. M1-, M3- and M5-muscarinic receptors) can protect cells from apoptosis following DNA damage, whereas those coupled to Gi-proteins (M2- and M4-muscarinic receptors) have no protective properties [6]. Our studies were consistent with those performed on a number of immortalized cell lines [8–10] and primary neuronal cultures [11,12], which have reported a pro-survival response for both the M1- and M3-muscarinic receptors. In fact, the anti-apoptotic effect of muscarinic receptors in neuronal models has led to the suggestion that this process may play a role in neuronal development, and lesions in cholinergic innervation observed in neurodegenerative diseases such as Alzheimer's may result in the loss of the survival stimulus that contributes to neuronal cell death.
Despite the fact that the pro-survival response of muscarinic receptors has been well-documented, the signalling mechanism that mediates this response is poorly understood. We have determined that a poly-basic region in the C-terminal tail of the M3-muscarinic receptor, which is conserved amongst the members of the Gq/11-coupled muscarinic receptor subtypes, is involved in coupling the receptors to the anti-apoptotic pathway [6]. The signalling mechanism appears not to involve phosphorylation of the receptor, activation of the mitogen-activated protein kinase pathways [ERK (extracellular-signal-regulated kinase) 1/2 or Jun kinase] or the stimulation of the pro-survival PKB (protein kinase B)/Akt pathway [6]. In the present study, we extend this work and investigate the possibility that the M3-muscarinic receptor anti-apoptotic response is mediated by the calcium/PLC (phospholipase C) pathway, activation of transcription and the up-regulation of the pro-survival Bcl-2 protein.
MATERIALS AND METHODS
Materials
Etoposide, U73122 and actinomycin D were from Calbiochem (Nottingham, U.K.). DEVD-pNA (Asp-Glu-Val-Asp-p-nitroanilide) was purchased from Bachem (St Helens, U.K.). All tissue culture reagents were purchased from Gibco BRL (Glasgow, U.K.). [Arg8]-vasopressin and carbachol were purchased from Sigma (Poole, U.K.). Anti-Bcl-2 antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.). [3H]UTP and myo-[3H]inositol were purchased from (Amersham Biosciences, Little Chalfont, Bucks., U.K.). The specific activity of [3H]UTP and myo-[3H]inositol were 15.3 Ci/mmol and 86 Ci/mmol respectively. All other chemicals were research grade and purchased from Fisher (Loughborough, U.K.).
Tissue culture
CHO (Chinese-hamster ovary) cells were transfected with cDNA encoding for the wild-type human M2- or M3-muscarinic receptors, and stably transfected clones were obtained by G418 sulphate selection. CHO cells stably expressing the M2-muscarinic receptor (CHO-M2 cells) at 0.75 pmol/mg of protein and CHO cells stably expressing the M3-muscarinic receptor (CHO-M3 cells) at 0.40 pmol/mg of protein were chosen for further study. CHO-M3 cells were maintained in α-MEM (α-minimal essential medium), supplemented with 10% (v/v) FCS (foetal calf serum), 100 units/ml penicillin/streptomycin, 2.5 μg/ml fungizone and 250 μg/ml G418 sulphate. CHO-M2 and human SHSY-5Y neuroblastoma cells were maintained in medium lacking G418 sulphate. CHO cells stably expressing the vasopressin V1A receptor (CHO-V1A) were provided by Dr Mark Wheatley (School of Biosciences, University of Birmingham, Birmingham, U.K.). CHO-V1A-expressing CHO cells were maintained in α-MEM, supplemented with 10% (v/v) FCS, 100 units/ml penicillin/streptomycin, 2.5 μg/ml fungizone and 250 μg/ml G418 sulphate.
Cell treatments in apoptosis experiments
Cells were plated at a density of (0.2–0.4)×106 cells per 10 cm2 plate 24 h before the start of the experiment. This resulted in plates that were 50–70% confluent. Apoptosis was induced by the addition of etoposide at a concentration of 250 μM or 25 μM for CHO cells and SH-SY5Y cells respectively, for periods of 6–24 h (usually 16 h). The etoposide treatment was carried out in cell culture medium. The ability of GPCRs to protect or enhance etoposide-induced apoptosis was assessed by including either the muscarinic agonist carbachol (1 mM) or the vasopressin receptor agonist [Arg8]-vasopressin (1 μM) with the etoposide treatment. The ability of the muscarinic receptor antagonist, atropine, to block the carbachol response was determined by the inclusion of atropine (0.5 μM) together with carbachol and etoposide.
In experiments to test the role of PLC in the anti-apoptotic effect of the M3-muscarinic receptor, CHO-M3 cells were treated with the PLC inhibitor U73122 (10 μM) for 30 min. The cells were then carefully washed three times with α-MEM and then treated with etoposide (250 μM) in the presence or absence of carbachol (1 mM) for 16 h.
The effect of the transcriptional inhibitor actinomycin D on the muscarinic receptor anti-apoptotic response was tested in CHO-M3 by pre-incubation with actinomycin D (100 ng/ml) for 30 min. In the continued presence of actinomycin D, cells were then treated with etoposide (250 μM) in the presence or absence of carbachol (1 mM) for 16 h.
Caspase assay
Following apoptosis induced by etoposide treatment (see above), floating and attached cells were harvested and pooled in PBS/0.5 mM EDTA, and cells were centrifuged at 500 g for 5 min in a bench-top centrifuge. The pellets were resuspended in 1 ml of lysis buffer [50 mM Hepes, pH 7.4, 1% CHAPS, 1 mM DTT (dithiothreitol) and 0.1 mM EDTA] and left on ice for 10 min. The cell lysates were cleared by centrifugation at 10000 g for 1 min. A Bradford assay [12a] was performed on the lysate, and 200–400 μg of cell lysate protein were diluted 1:2 (v/v) in reaction buffer [50 mM Hepes, pH 7.4, 100 mM NaCl, 0.1% CHAPS, 10 mM DTT, 1 mM EDTA and 10% (v/v) glycerol] in plastic 96-well plates. A 1:10 (v/v) dilution of 4 mM DEVD-pNA was added to each well of the 96-well plate and the plate was incubated at 37 °C for 2–4 h. Cleavage of the DEVD-pNA substrate was monitored colorimetrically at 405 nm in a microplate reader.
Measurement of gene transcription
Total gene transcription was determined by measurement of [3H]UTP incorporation into CHO-M3 cells grown in 6-well plates. At 24 h before the start of the experiment, cells were plated at a density of approx. 0.8×105 cells/well which gave a confluency of 50–70% at the start of the experiment. Cells were serum-starved in KHB (Krebs/Hepes buffer) (10 mM Hepes, pH 7.4, 118 mM NaCl, 1.17 mM KHPO3, 4.3 mM KCl, 1.17 mM MgSO4·7H2O, 1.3 mM CaCl2, 25 mM NaHCO3 and 11.7 mM glucose) for 2 h, after which the medium was aspirated and replaced with 1 ml of KHB containing 1 μCi/ml of [3H]UTP per well. Cells were stimulated with carbachol (1 mM) or 10% FCS for times ranging from 0.5–60 min. Reactions were terminated by the addition of 2 μM atropine sulphate. Cells were then washed twice with ice-cold PBS and twice with 6% trichloroacetic acid. Cells were then lysed in 0.1M NaOH, 0.1% (w/v) SDS and the amount of [3H]uridine incorporation was determined by scintillation counting.
In experiments to test the effects of the transcriptional inhibitor actinomycin D, CHO-M3 cells seeded on 6-well plates were serum-starved for 2 h, and were then incubated with or without actinomycin D (100 ng/ml) for 30 min before the addition of [3H]UTP (1 μCi/ml). Cells were then treated with carbachol (1 mM) for 30 min, and the reaction was terminated and [3H]UTP incorporation was determined as described above.
Measurement of total [3H]inositol phosphate production
CHO-M3 or CHO-V1A cells were plated on to 24-well plates 24 h before the experiment. At the start of the experiment, the plates were 50% confluent. myo-[3H]inositol (3 μCi/ml) was added per well, and cells were incubated for 48 h at 37 °C. LiCl (10 mM) was added for 15 min before agonist application. Cells were then stimulated with agonist [i.e. carbachol (1 mM) or [Arg8]-vasopressin (1 μM)] for 25 min, and reactions were terminated by the addition of ice-cold trichloroacetic acid. EDTA (250 μM) was added to each sample, and total inositol phosphates were then extracted in tri-n-octylamine/1,1,2-trichloro-trifluoroethane (1:1, v/v). After extraction, samples were neutralized with 250 mM NaHCO3. Inositol phosphates were then purified on Dowex columns and eluted with 1 M hydrochloric acid after washing with 25 mM ammonium formate as described previously [13]. The amount of [3H]inositol phosphate was then determined by scintillation counting.
Western blotting
CHO-M3 cells were plated on to 10 cm2 plates as for the apoptosis experiments (see above). Apoptosis was induced by treatment with etoposide (250 μM) for 16 h (see above). The experiment was terminated by harvesting floating and adhered cells in PBS/0.5 mM EDTA solution. The cell pellets obtained by centrifuging at 500 g for 3 min were resuspended in caspase lysis buffer, and lysates were placed on ice for 10 min. Lysates were pre-cleared by centrifugation at 10000 g for 1 min, and the pellet was discarded. Bradford assays [12a] were performed on the lysates, and 100 μg of protein/well was electrophoresed by SDS/PAGE (12% gels). Proteins were transferred on to nitrocellulose and blocked in T-TBS (Tween/Tris-buffered saline) [0.1% (v/v) Tween 20, 0.15 M NaCl, 10 mM Tris/HCl, pH 7.4] containing 10% (w/v) dried milk. The nitrocellulose was probed with Bcl-2 antisera (1:500 dilution), followed by three washes with T-TBS. Horseradish-peroxidase-conjugated anti-mouse secondary antibody was added (1:1000 dilution), followed by three washes in T-TBS. Proteins were visualized by ECL® (enhanced chemiluminescence; Amersham Biosciences) and fluorography.
To assess the ability of muscarinic receptor stimulation to regulate Bcl-2 expression during apoptosis CHO-M3 cells were treated with carbachol (1 mM) at the same time as the etoposide addition. After 2 min, atropine (0.5 μM) was added to antagonize the muscarinic receptor response. The treatment (i.e. in the presence of etoposide, carbachol and atropine) was then allowed to continue for 16 h (overnight). In parallel experiments, cells were treated with etoposide in the absence or presence of carbachol for 16 h with no addition of atropine. The experiment was terminated by lysis of the cells as described above.
Intracellular calcium measurements
CHO cells grown on 22 mm coverslips were loaded with 5 μM fura-2 acetoxymethyl-ester for 60 min at room temperature (22 °C). Measurement of [Ca2+]i (intracellular calcium concentration) was performed as described previously [14]. The ability of the PLC inhibitor U73122 to inhibit the muscarinic calcium response was tested by the addition of U73122 (10 μM) 30 min before a challenge of carbachol (1 mM) stimulation.
RT (reverse transcriptase)-PCR of Bcl-2
CHO-M3 cells were plated on to 10 cm2 plates and were treated with or without etoposide (250 μM) for 16 h as described for the apoptosis experiments (see above). The effect of muscarinic receptor stimulation on the etoposide-treated cells was assessed by including carbachol (1 mM) together with etoposide. The reaction was stopped by harvesting and pooling the floating and adhered cells in PBS/0.5 mM EDTA and extracting the total RNA from the cell pellet using RNeasy (Qiagen, Crawley, West Sussex, U.K.). The RNA (400 μg) was reverse-transcribed using SuperScript III enzyme (Invitrogen, Carlsbad, CA, U.S.A.). The resulting cDNA was used in conjunction with SYBR® Green mastermix (Applied Biosystems, Warrington, Cheshire, U.K.) to optimize the primer conditions for Bcl-2 and β-actin using an ABI PRISM 7700 RT-PCR machine. Primers for RT-PCR were designed using Primer Express software v2.0 (Applied Biosystems) against the mouse sequence of Bcl-2 and β-actin. Bcl-2 forward primer 5′-GGATCCAGGATAACGGAGGC-3′; reverse primer 3′-ACAGCCAGGAGAAATCAAACAGA-5′, and β-actin forward primer 5′-GATTACTGCTCTGGCTCCTAGCA-3′ and reverse primer 3′-GTGGACAGTGAGGCCAGGAT-5′. Following RT-PCR, the Bcl-2 levels were normalized to that of β-actin. Importantly, the transcription levels recorded for β-actin were not significantly affected by etoposide treatment nor was it affected by etoposide and carbachol treatment.
RESULTS
The involvement of the calcium/PLC pathway in the M3-muscarinic receptor anti-apoptotic response
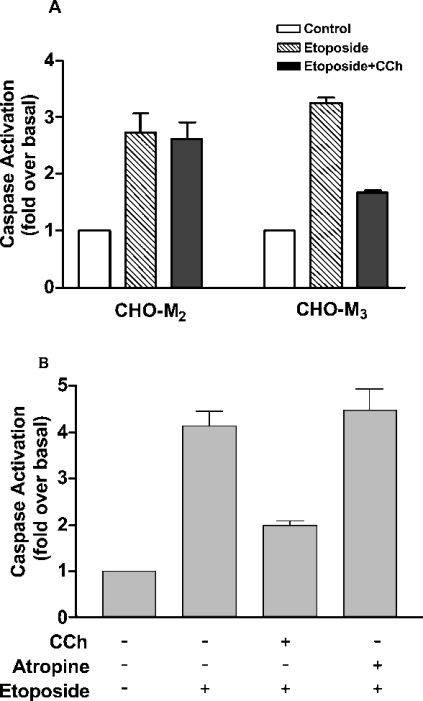
Apoptosis in CHO cells can be induced by an overnight treatment with the topoisomerase II inhibitor etoposide (250 μM). We have shown previously that this results in an increase in a number of markers of apoptosis, including elevation of annexin V labelling of cell-surface phosphatidylserine, DNA fragmentation and increased caspase activity [6,7]. Consistent with these earlier studies, we show here that etoposide induces an approx. 3 fold increase in caspase activation in CHO cells (Figure 1). The increase in caspase activity can be attenuated in CHO cells expressing the M3-muscarinic receptor (CHO-M3 cells) by treatment with the muscarinic agonist carbachol (Figure 1A). In agreement with our earlier work, this muscarinic anti-apoptotic effect was restricted to the Gq/11-coupled muscarinic receptor subtypes, since attenuation of caspase activity was not observed on stimulation of muscarinic receptors in CHO cells expressing the Gi-coupled M2-muscarinic receptor subtype (Figure 1A). Importantly, the anti-apoptotic effect of the M3-muscarinic receptor is not exclusive to CHO cells, since muscarinic receptor stimulation of the human neuroblastoma cell line SHSY-5Y, where the M3-muscarinic receptor is endogenously expressed, also mediates protection from etoposide-induced cell death (Figure 1B).
Figure 1. Attenuation of etoposide-mediated caspase activation by M3-muscarinic receptors in CHO-M3 and SHSY-5Y cells.
(A) CHO-M2 and CHO-M3 cells seeded on 10 cm2 plates were treated with vehicle (Control), carbachol (CCh; 1 mM) and/or etoposide (250 μM) for 16 h, and cell lysates were processed for caspase activity. (B) SHSY-5Y neuroblastoma cells seeded on 10 cm2 plates were treated with vehicle, carbachol (CCh; 1 mM), etoposide (25 μM) and/or atropine (0.5 μM) for 16 h, and cell lysates were processed for caspase assays. Results are means±S.E.M. (n=3).
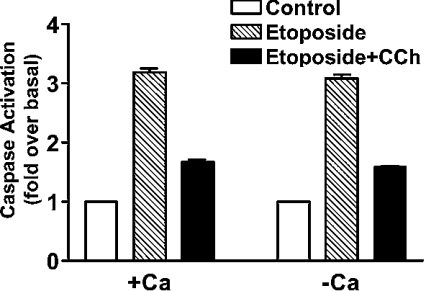
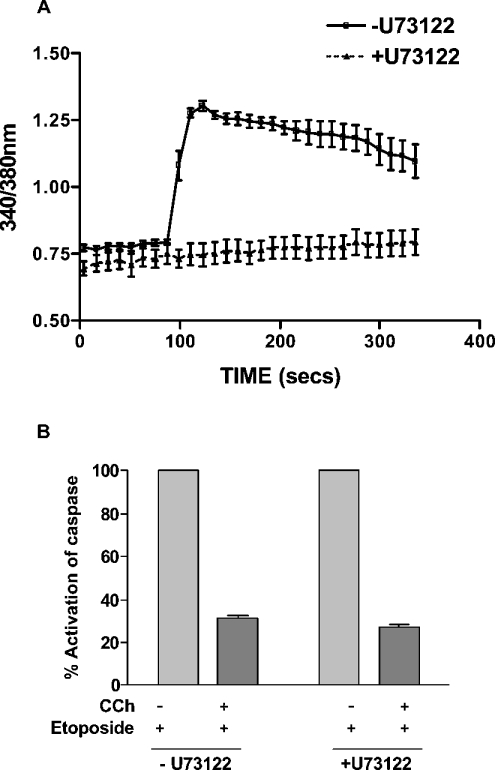
In studies designed to investigate the signalling mechanism of the muscarinic anti-apoptotic response, we investigated the role of the calcium/PLC signalling pathway. This seemed particularly relevant, since only those muscarinic receptor subtypes that are coupled to the calcium/PLC pathway showed an anti-apoptotic response in CHO cells [6]. We have reported previously that pre-incubation of CHO-M3 cells in a calcium-free buffer containing 3 mM EGTA reduced the peak rise in intracellular free calcium following M3-muscarinic receptor stimulation and removed completely the plateau phase of the calcium response [15]. Despite this disruption to the calcium response, removal of extracellular calcium in this way had no significant effect on the muscarinic anti-apoptotic response in CHO-M3 cells (Figure 2). Furthermore, inhibition of PLC activity with U73122 resulted in an ablation of the calcium response to M3-muscarinic receptor stimulation, but did not affect the anti-apoptotic response (Figure 3). These data indicate that the M3-muscarinic receptor anti-apoptotic response was independent of the calcium/PLC signalling pathway.
Figure 2. The M3-muscarinic receptor anti-apoptotic response is independent of extracellular calcium.
CHO-M3 cells were seeded on 10 cm2 plates. Cells were incubated in Krebs buffer (+calcium) or in Krebs buffer lacking calcium and supplemented with 3 mM EDTA (−calcium) for 3 min. CHO-M3 cells were treated with carbachol (CCh; 1 mM) for 5 min and cells were washed three times with α-MEM. CHO-M3 cells were then incubated for 16 h with etoposide (250 μM) and cell lysates processed for caspase activity. Results are means±S.E.M. (n=3).
Figure 3. Muscarinic M3-mediated inhibition of etoposide-induced caspase activation is unaffected by the PLC inhibitor U73122.
(A) CHO-M3 cells were seeded on to 25 mm glass coverslips and loaded with 5 μM fura-2. U73122 (10 μM) or vehicle was added for 30 min before a challenge with carbachol (1 mM) stimulation. The results shown are the means±S.E.M. of at least ten independent cells. (B) CHO-M3 cells seeded on 10 cm2 plates were treated with U73122 (10 μM) for 30 min, and the cells were then carefully washed three times with α-MEM. Cells were then treated with or without carbachol (CCh; 1 mM) and etoposide (250 μM) for 16 h. Floating and attached cells were harvested and pooled, and cell lysates were processed for caspase activity, as described in the Materials and methods section. Results are means±S.E.M. (n=3).
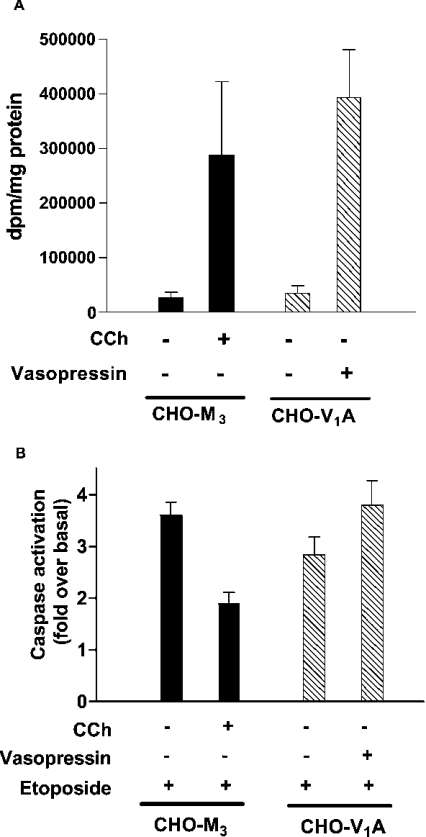
This conclusion was supported further in studies on the Gq/11-coupled vasopressin V1A receptor. This receptor stably expressed in CHO cells gave a robust inositol phosphate response similar to that observed with the M3-muscarinic receptor (Figure 4A). However, in contrast with the M3-muscarinic receptor, the V1A receptor was unable to mediate an anti-apoptotic response (Figure 4B).
Figure 4. Both M3-muscarinic and vasopressin V1A receptors increase inositol phosphate production, but only the M3-muscarinic receptor mediates an anti-apoptotic response.
(A) CHO-M3 and CHO-V1A cells plated on 24-well plates were stimulated with carbachol (CCh; 1 mM) or vasopressin (1 μM) for 25 min. The reactions were terminated with 1 M trichloroacetic acid and inositol phosphate production was determined as described in the Materials and methods section. (B) CHO-M3 and CHO-V1A cells were stimulated with carbachol (CCh; 1 mM) or vasopressin (1 μM) and etoposide (250 μM). Cells were incubated for 16 h at 37 °C. Floating and attached cells were harvested and pooled, and cell lysates were processed for caspase activity as described in the Materials and methods section. Results are means±S.E.M. (n=3).
The involvement of transcription in the M3-muscarinic receptor anti-apoptotic response
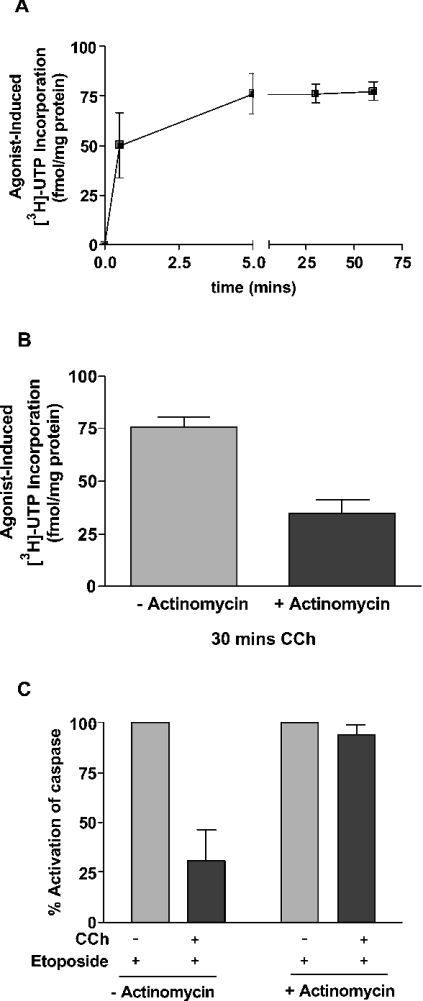
One important feature of the M3-muscarinic receptor anti-apoptotic response is that a short stimulation (as little as 30 s) is sufficient to protect CHO-M3 cells from apoptosis induced by etoposide [6]. This indicates that the signalling process involved must be rapid in onset and maintained for many hours, even after the removal of the muscarinic agonist. In the present study, we investigated the possibility that changes in transcription may contribute to the mechanism of the muscarinic receptor protective response. Total cellular transcription was determined by the incorporation of [3H]UTP, and following M3-muscarinic receptor stimulation, this was observed to rapidly increase within the first 30 s of agonist stimulation (50.0±16.2 fmol/mg of protein; n=3, S.E.M.) and then be maintained for at least 60 min (Figure 5A). The increase in transcription could be attenuated by 54.5±8.8% (n=3, S.E.M.) by pre-incubation with the transcriptional inhibitor actinomycin D (Figure 5B). The concentration of actinomycin D used in the present study was determined by careful titration to establish a concentration (e.g. 100 ng/ml) that was sufficient to give an inhibition of transcription (Figure 5B), but not high enough to give an apoptotic effect in its own right. At this concentration actinomycin D was found to completely attenuate the M3-muscarinic receptor anti-apoptotic response (Figure 5C), indicating that transcription was important in muscarinic-receptor-mediated cell survival.
Figure 5. The M3-muscarinic-receptor-mediated anti-apoptotic response and transcriptional activation are both attenuated by actinomycin D treatment.
(A) CHO-M3 cells seeded on to 6-well plates were serum-starved for 2 h before the addition of [3H]UTP (1 μCi/ml). Cells were then treated with carbachol (1 mM) for the indicated times. [3H]UTP incorporation was determined by scintillation counting. Data shown is the agonist-induced incorporation of [3H]UTP subtracted from that observed in the absence of agonist, and represent the means±S.E.M. (n=3). (B) CHO-M3 cells seeded on 6-well plates were serum-starved for 2 h and then incubated with or without actinomycin D (100 ng/ml) for 30 min before the addition of [3H]UTP (1 μCi/ml). Cells were then treated with carbachol (1 mM) for 30 min. [3H]UTP incorporation was determined by scintillation counting. Results are means±S.E.M. (n=3). (C) CHO-M3 cells seeded on to 10 cm2 plates were pre-incubated with or without actinomycin D (100 ng/ml) for 30 min. Cells were then treated with carbachol (CCh; 1 mM) and/or etoposide (250 μM) for 16 h and cell lysates processed for caspase activity. Caspase activity of 100% is given as the increase in caspase activity in the presence of etoposide. Results are means±S.E.M. (n=3).
M3-muscarinic receptor stimulation up-regulates the anti-apoptotic Bcl-2 protein
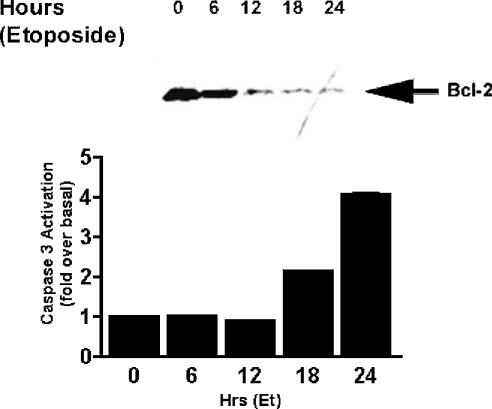
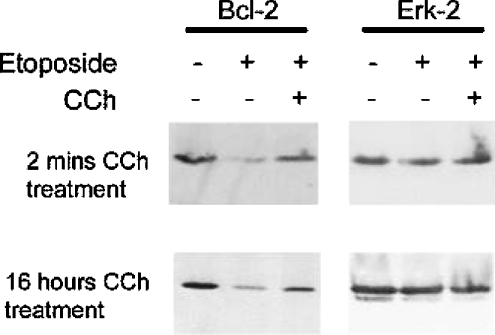
The members of the Bcl-2 protein family are widely thought to represent a central point of regulation of caspase-mediated apoptosis. The balance of pro- and anti-apoptotic members of the Bcl-2 family, particularly in relation to their mitochondrial membrane localization, determines whether a cell enters apoptosis (see the Discussion). In the present study, we show that levels of the anti-apoptotic protein, Bcl-2, fall dramatically following etoposide treatment (Figure 6). The fall in Bcl-2 levels correlate closely with an increase in caspase activity (Figure 6). Muscarinic receptor stimulation of CHO-M3 cells was able to significantly prevent the reduction in Bcl-2 expression levels induced by etoposide (Figure 7). The ability of the M3-muscarinic receptor to prevent the fall in Bcl-2 levels following etoposide treatment was evident following either a transient or prolonged application of the muscarinic receptor agonist (Figure 7). Interestingly, levels of Bcl-2 were not affected by muscarinic receptor stimulation in CHO-M3 cells that were not induced to undergo apoptosis (results not shown). These data demonstrate a close relationship between the concentration of cellular Bcl-2 protein and cell survival, and suggests that at least one mechanism employed by the M3-muscarinic receptor to protect cells from apoptosis is to prevent a fall in Bcl-2 protein levels mediated by an apoptotic stimulus.
Figure 6. Time-course of etoposide-induced decrease in Bcl-2 levels and caspase activation.
CHO-M3 cells seeded on 10 cm2 plates were incubated with etoposide (250 μM) for the times indicated. Cell lysates were prepared and run on a SDS/PAGE (12% gels) and Western blotted for Bcl-2. Data shown is representative of three separate experiments (upper panel). In parallel experiments, a time course for caspase activity was determined following etoposide (Et; 250 μM) treatment for the indicated times. Results for caspase activation are means±S.E.M. (n=3) (lower panel).
Figure 7. The decrease in the expression levels of the anti-apoptotic Bcl-2 protein following incubation of cells with etoposide can be inhibited by activation of the M3-muscarinic receptor.
CHO-M3 cells seeded on to 10 cm2 plates were incubated with or without carbachol (CCh; 1mM) either for 2 min before a 16-h treatment with etoposide (250 μM) or incubated with etoposide and carbachol for 16 h. In the case of cells treated with carbachol for 2 min, the carbachol stimulation was stopped by the addition of the muscarinic receptor antagonist atropine (0.5 μM) followed by three washes in α-MEM before being incubated with etoposide for 16 h. Cell lysates were then prepared and separated by SDS/PAGE (12% gels; 100 μg of protein/well). The gel was then probed for Bcl-2 immunoreactivity. The nitrocellulose was then stripped and re-probed with ERK antisera in order to check for equal lane loading (right-hand panels). Gels are representative of three separate experiments.
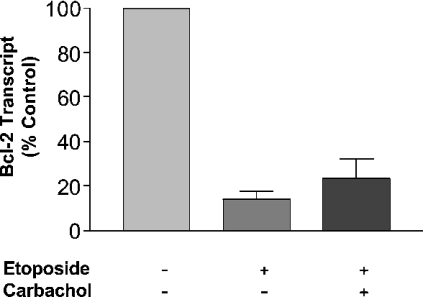
To investigate this phenomenon further, RT-PCR studies were conducted, which demonstrated that etoposide treatment substantially reduced Bcl-2 mRNA levels, but that muscarinic receptor stimulation did not significantly protect against this fall in the Bcl-2 transcript (Figure 8). This suggests that although the fall in Bcl-2 expression mediated by etoposide could be explained, at least in part, by a decrease in Bcl-2 mRNA, the ability of muscarinic receptor stimulation to protect against this fall in Bcl-2 expression is not at the level of transcription, but may be at the level of stabilization of the Bcl-2 protein. We are currently testing the hypothesis that muscarinic receptor stimulation in etoposide treated cells can increase the half-life of the Bcl-2 protein.
Figure 8. Decreased mRNA levels of Bcl-2 following etoposide treatment could not be inhibited by muscarinic receptor stimulation in CHO-M3 cells.
CHO-M3 cells seeded on to 10 cm2 plates were incubated with vehicle or etoposide (250 μM) for 16 h in the absence or presence of carbachol (1 mM). Total RNA was then extracted and reverse transcribed. RT-PCR using primers specific for Bcl-2 and the internal control β-actin was performed using SYBR® Green mastermix. Levels of the Bcl-2 transcript were then normalized to β-actin and expressed as a percentage of the control vehicle-treated cells. Results are means±S.E.M. (n=3).
DISCUSSION
Apoptosis occurs widely in development and tissue maintenance and has been linked with certain pathologies particularly related to neurodegeneration [16,17]. Whereas the biochemical pathways that result in apoptosis are becoming clearer, the mechanisms that provide points of regulation in the apoptotic process are just emerging. A recent development in this field is the realization that GPCRs are able to regulate apoptosis in either a positive or a negative manner, depending on the receptor subtype and cellular environment. The large number of GPCR subtypes, and their wide and complex tissue distribution suggests that GPCRs could provide a sophisticated mechanism of fine-tuning the apoptotic process. The molecular mechanisms by which GPCRs couple to the apoptotic pathway are, however, poorly understood. In the present study, we focus on the anti-apoptotic response of the M3-muscarinic receptor, and show that this response is not linked to the ability of the receptor to signal through the calcium/PLC pathway, but does appear to involve gene transcription and changes in the level of the anti-apoptotic Bcl-2 protein.
We have reported previously that the muscarinic-mediated cell-survival response in CHO cells is restricted to those subtypes of the muscarinic receptor family that are coupled to Gq/11-proteins (e.g. M1, M3 and M5) and hence the calcium/PLC pathway [6]. It would seem likely, therefore, that this pathway would be involved in the muscarinic anti-apoptotic response. However, disruption of calcium signalling by removal of extracellular calcium or inhibition of PLC activity with the selective PLC inhibitor U73122 had no effect on the ability of the M3-muscarinic receptor to mediate the anti-apoptotic response. These data are consistent with our previous report that a C-terminal tail mutant of the M3-muscarinic receptor that was unable to mediate a cell-survival response was nevertheless still able to couple to the calcium/PLC pathway with equivalent efficiency as the wild-type receptor [6]. Furthermore, the Gq/11-coupled vasopressin V1A receptor was unable to mediate an anti-apoptotic response in CHO cells, despite coupling efficiently to the PLC pathway. Our studies, therefore, demonstrate that the anti-apoptotic mechanism observed here for the M3-muscarinic receptor does not involve calcium/PLC signalling.
These findings add to our earlier studies that have eliminated a role for the mitogen-activated protein kinases, ERK1/2, Jun N-terminal kinase and p38, as well as the PKB/Akt pathway, in the muscarinic receptor anti-apoptotic response [6]. Furthermore, when considering possible mechanisms for cell survival mediated by the M3-muscarinic receptor, it is important to note that only a transient pulse of muscarinic agonist (as short as 30 s) is necessary to protect cells from an overnight exposure to etoposide [6]. The mechanism that is in operation must, therefore, be rapid in onset and initiate a process that is maintained even after the agonist is withdrawn.
In order to probe further the possible molecular mechanisms of the muscarinic receptor cell-survival response, we investigated the role of gene transcription. In the present study, we show that a 30 s pulse of carbachol was sufficient to induce a significant increase in gene transcription in CHO-M3 cells. The increase in gene transcription could be attenuated with the transcriptional inhibitor, actinomycin D. The inhibitory effect of actinomycin D on gene transcription correlated with its ability to inhibit the M3-muscarinic receptor anti-apoptotic response, suggesting that up-regulation of gene expression is intimately linked with the ability of the M3-muscarinic receptor to inhibit programmed cell death. It is of importance to note that rapid gene transcription induced by muscarinic receptors has been reported previously, and the genes identified include a number of immediate-early genes such as CYR61 [18], c-fos, c-jun [19,20], c-myc [21] and AP-1 [22]. Up-regulation of c-fos and CYR61 is dependent on intracellular calcium [18,19], whereas up-regulation of c-myc, c-jun and AP-1 occurs in the absence of changes in intracellular calcium [19,21,22]. It is also of note that the M3-muscarinic receptor requires only a very transient pulse of carbachol (approx. 1.5 min) to induce a maximal up-regulation of c-fos and c-jun expression [19]. These previous studies confirm that M3-muscarinic receptors possess the capability to rapidly and selectively up-regulate gene transcription in a calcium-independent manner. It is possible, therefore, that rapid gene transcription, independent of calcium/PLC signalling (and inhibited by actinomycin D) could underlie the anti-apoptotic process that is mediated by the M3-muscarinic receptor in CHO-M3 cells.
Central to the molecular mechanism of apoptosis is the activation of the caspase cascade [23]. Many apoptotic stimuli initiate caspase activity by altering the balance of pro- and anti-apoptotic members of the Bcl-2 family in the outer mitochondrial membrane [24]. Pro-apoptotic Bcl-2 proteins, such as Bax and Bad, are present in the cytoplasm and translocate to the outer mitochondrial membrane in response to certain apoptotic stimuli. Once located to the mitochondria, they increase membrane permeability allowing for the release of cytochrome c into the cytoplasm [25–27]. Cytochrome c then forms a complex with Apaf-1 (apoptosis-activating factor-1) and pro-caspase 9 which results in the auto-catalytic processing of pro-caspase 9 and the subsequent activation of the downstream caspase cascade [28]. Anti-apoptotic Bcl-2 family members, such as Bcl-2 and Bcl-xL, are constitutively present in the mitochondrial membrane and can block apoptosis by preventing the formation of the mitochondrial permeability transition pore and thereby the release of cytochrome c [26]. Hence one of the key features in cell survival is the balance between the expression levels of the pro- and anti-apoptotic Bcl-2 proteins and the translocation of the pro-apoptotic Bcl-2 proteins to the mitochondrial membrane.
In the present study, we demonstrate that etoposide-induced caspase activation correlates with a decrease in the expression levels of the anti-apoptotic protein, Bcl-2. This fall in Bcl-2 levels can be attenuated by either a transient or prolonged stimulation of the M3-muscarinic receptor. Hence the ability of the M3-muscarinic receptor to protect cells from etoposide-induced cell death appears to correlate with the up-regulation of the anti-apoptotic Bcl-2 protein.
RT-PCR studies demonstrated that the fall in Bcl-2 expression following etoposide treatment correlated with a fall in the levels of the Bcl-2 transcript. Stimulation of the M3-muscarinic receptor did not, however, prevent the fall in Bcl-2 mRNA levels. It appears, therefore, that the ability of the M3-muscarinic receptor to maintain the expression levels of Bcl-2 in cells induced into apoptosis by etoposide is not linked to an ability to protect from an etoposide-induced decrease in Bcl-2 transcription. The possibility that the M3-muscarinic receptor can increase the stability of the Bcl-2 protein under apoptotic conditions is currently being pursued.
The RT-PCR studies also highlight the complexity of the protective response that is mediated by the M3-muscarinic receptor. By the use of actinomycin D, we demonstrate a role for transcription in the protective response of the M3-muscarinic receptor, but then in the case of the regulation of anti-apoptotic Bcl-2 protein the M3-muscarinic receptor increases protein expression levels via a mechanism that appears unrelated to gene transcription. These data suggest that the ability of the M3-muscarinic receptor to protect cells from apoptosis is likely to be a multifaceted phenomenon.
The muscarinic anti-apoptotic response has been identified in a number of stable cell lines and primary neuronal cultures [8,11,12]. In particular, M3-muscarinic receptors in cerebellar primary neuronal cultures can protect against cell death induced by culturing in non-depolarizing conditions [11]. Furthermore, in the human neuroblastoma cell line, SHSY-5Y, M3-muscarinic receptor stimulation has been previously reported to protect from apoptosis induced following oxidative stress by preventing the translocation of the pro-apoptotic proteins, Bax and Bad, to the mitochondria [9]. Our studies demonstrating that the M3-muscarinic receptor can protect both CHO and SHSY-5Y cells from etoposide-induced cell death support the notion that the cell-survival response of the M3-muscarinic receptor is associated with a number of different cell types. Understanding the biochemical mechanism of this protective response will aid in the ultimate goal of determining the physiological importance of the M3-muscarinic receptor anti-apoptotic response.
Acknowledgments
We thank the Wellcome Trust (grant 047600) for their financial support and Dr Mark Wheatley and Dr Stuart Hawtin for the gift of the V1A-expressing cell line. We also thank Tim Gant for help with the RT-PCR.
References
- 1.Marinissen M. J., Gutkind J. S. G-protein-coupled receptors and signaling networks: emerging paradigms. Trends Pharmacol. Sci. 2001;22:368–376. doi: 10.1016/s0165-6147(00)01678-3. [DOI] [PubMed] [Google Scholar]
- 2.Zhu W. Z., Zheng M., Koch W. J., Lefkowitz R. J., Kobilka B. K., Xiao R. P. Dual modulation of cell survival and cell death by β2-adrenergic signaling in adult mouse cardiac myocytes. Proc. Natl. Acad. Sci. U.S.A. 2001;98:1607–1612. doi: 10.1073/pnas.98.4.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen C. K., Burns M. E., Spencer M., Niemi G. A., Chen J., Hurley J. B., Baylor D. A., Simon M. I. Abnormal photoresponses and light-induced apoptosis in rods lacking rhodopsin kinase. Proc. Natl. Acad. Sci. U.S.A. 1999;96:3718–3722. doi: 10.1073/pnas.96.7.3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi S., Hao W., Chen C. K., Simon M. I. Gene expression profiles of light-induced apoptosis in arrestin/rhodopsin kinase-deficient mouse retinas. Proc. Natl. Acad. Sci. U.S.A. 2001;98:13096–13101. doi: 10.1073/pnas.201417498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dale L. B., Bhattacharya M., Anborgh P. H., Murdoch B., Bhatia M., Nakanishi S., Ferguson S. S. G protein-coupled receptor kinase-mediated desensitization of metabotropic glutamate receptor 1A protects against cell death. J. Biol. Chem. 2000;275:38213–38220. doi: 10.1074/jbc.M006075200. [DOI] [PubMed] [Google Scholar]
- 6.Budd D. C., McDonald J., Emsley N., Cain K., Tobin A. B. The C-terminal tail of the M3-muscarinic receptor possesses anti-apoptotic properties. J. Biol. Chem. 2003;278:19565–19573. doi: 10.1074/jbc.M211670200. [DOI] [PubMed] [Google Scholar]
- 7.Tobin A. B., Budd D. C. The anti-apoptotic response of the Gq/11-coupled muscarinic receptor. Biochem. Soc. Trans. 2003;31:1182–1185. doi: 10.1042/bst0311182. [DOI] [PubMed] [Google Scholar]
- 8.Lindenboim L., Pinkas-Kramarski R., Sokolovsky M., Stein R. Activation of muscarinic receptors inhibits apoptosis in PC12M1 cells. J. Neurochem. 1995;64:2491–2499. doi: 10.1046/j.1471-4159.1995.64062491.x. [DOI] [PubMed] [Google Scholar]
- 9.De Sarno P., Shestopal S. A., King T. D., Zmijewska A., Song L., Jope R. S. Muscarinic receptor activation protects cells from apoptotic effects of DNA damage, oxidative stress, and mitochondrial inhibition. J. Biol. Chem. 2003;278:11086–11093. doi: 10.1074/jbc.M212157200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leloup C., Michaelson D. M., Fisher A., Hartmann T., Beyreuther K., Stein R. M1 muscarinic receptors block caspase activation by phosphoinositide 3-kinase- and MAPK/ERK-independent pathways. Cell Death Differ. 2000;7:825–833. doi: 10.1038/sj.cdd.4400713. [DOI] [PubMed] [Google Scholar]
- 11.Yan G. M., Lin S. Z., Irwin R. P., Paul S. M. Activation of muscarinic cholinergic receptors blocks apoptosis of cultured cerebellar granule neurons. Mol. Pharmacol. 1995;47:248–257. [PubMed] [Google Scholar]
- 12.Koh J. Y., Palmer E., Cotman C. W. Activation of the metabotropic glutamate receptor attenuates N-methyl-D-aspartate neurotoxicity in cortical cultures. Proc. Natl. Acad. Sci. U.S.A. 1991;88:9431–9435. doi: 10.1073/pnas.88.21.9431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12a.Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 13.Wojcikiewicz R. J., Lambert D. G., Nahorski S. R. Regulation of muscarinic agonist-induced activation of phosphoinositidase C in electrically permeabilized SH-SY5Y human neuroblastoma cells by guanine nucleotides. J. Neurochem. 1990;54:676–685. doi: 10.1111/j.1471-4159.1990.tb01924.x. [DOI] [PubMed] [Google Scholar]
- 14.Budd D. C., Rae A., Tobin A. B. Activation of the mitogen-activated protein kinase pathway by a Gq/11-coupled muscarinic receptor is independent of receptor internalization. J. Biol. Chem. 1999;274:12355–12360. doi: 10.1074/jbc.274.18.12355. [DOI] [PubMed] [Google Scholar]
- 15.Wojcikiewicz R. J., Tobin A. B., Nahorski S. R. Muscarinic receptor-mediated inositol 1,4,5-trisphosphate formation in SH-SY5Y neuroblastoma cells is regulated acutely by cytosolic Ca2+ and by rapid desensitization. J. Neurochem. 1994;63:177–185. doi: 10.1046/j.1471-4159.1994.63010177.x. [DOI] [PubMed] [Google Scholar]
- 16.Jacobson M. D., Weil M., Raff M. C. Programmed cell death in animal development. Cell. 1997;88:347–354. doi: 10.1016/s0092-8674(00)81873-5. [DOI] [PubMed] [Google Scholar]
- 17.Gasic G. P., Nicotera P. To die or to sleep, perhaps to dream. Toxicol. Lett. 2003;139:221–227. doi: 10.1016/s0378-4274(02)00487-3. [DOI] [PubMed] [Google Scholar]
- 18.Albrecht C., von der Kammer H., Mayhaus M., Klaudiny J., Schweizer M., Nitsch R. M. Muscarinic acetylcholine receptors induce the expression of the immediate early growth regulatory gene CYR61. J. Biol. Chem. 2000;275:28929–28936. doi: 10.1074/jbc.M003053200. [DOI] [PubMed] [Google Scholar]
- 19.Trejo J., Brown J. H. c-fos and c-jun are induced by muscarinic receptor activation of protein kinase C but are differentially regulated by intracellular calcium. J. Biol. Chem. 1991;266:7876–7882. [PubMed] [Google Scholar]
- 20.Trejo J., Chambard J. C., Karin M., Brown J. H. Biphasic increase in c-jun mRNA is required for induction of AP-1-mediated gene transcription: differential effects of muscarinic and thrombin receptor activation. Mol. Cell. Biol. 1992;12:4742–4750. doi: 10.1128/mcb.12.10.4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blackshear P. J., Stumpo D. J., Huang J. K., Nemenoff R. A., Spach D. H. Protein kinase C-dependent and -independent pathways of proto-oncogene induction in human astrocytoma cells. J. Biol. Chem. 1987;262:7774–7781. [PubMed] [Google Scholar]
- 22.Chae H. D., Suh B. C., Joh T. H., Kim K. T. AP1-mediated transcriptional enhancement of the rat tyrosine hydroxylase gene by muscarinic stimulation. J. Neurochem. 1996;66:1264–1272. doi: 10.1046/j.1471-4159.1996.66031264.x. [DOI] [PubMed] [Google Scholar]
- 23.Cohen G. M. Caspases: the executioners of apoptosis. Biochem. J. 1997;326:1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adams J. M., Cory S. Life-or-death decisions by the Bcl-2 protein family. Trends Biochem. Sci. 2001;26:61–66. doi: 10.1016/s0968-0004(00)01740-0. [DOI] [PubMed] [Google Scholar]
- 25.Wolter K. G., Hsu Y. T., Smith C. L., Nechushtan A., Xi X. G., Youle R. J. Movement of Bax from the cytosol to mitochondria during apoptosis. J. Cell Biol. 1997;139:1281–1292. doi: 10.1083/jcb.139.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gross A., Jockel J., Wei M. C., Korsmeyer S. J. Enforced dimerization of BAX results in its translocation, mitochondrial dysfunction and apoptosis. EMBO J. 1998;17:3878–3885. doi: 10.1093/emboj/17.14.3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gross A., McDonnell J. M., Korsmeyer S. J. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- 28.Cain K., Bratton S. B., Cohen G. M. The Apaf-1 apoptosome: a large caspase-activating complex. Biochimie. 2002;84:203–214. doi: 10.1016/s0300-9084(02)01376-7. [DOI] [PubMed] [Google Scholar]