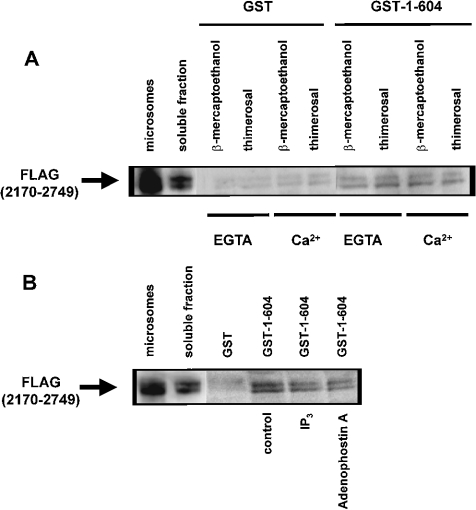
Figure 6. Effect of thimerosal and IP3 on the interaction between the IP3-binding and transmembrane domains of IP3R1.
(A) Pull-down experiment applied for GST–1–604 with FLAG(2170–2749) in the presence of 1 mM 2-mercaptoethanol or 100 μM thimerosal. EGTA (1 mM) or 25 μM Ca2+ was added as indicated in the Figure. The recombinant FLAG(2170–2749) was incubated with GST or GST–1–604 immobilized on glutathione–Sepharose 4B. After washing, the retained proteins were eluted. After SDS/PAGE, the proteins were analysed by immunoblotting with ANTI-FLAG® M2 monoclonal antibody. Lane ‘microsomes’ contains 0.5 μg of protein and lane ‘soluble fraction’ contains 2 μg of protein, whereas the lanes representing samples obtained after GST-pull down contain 15-fold more material than the soluble fraction. (B) Pull-down experiment applied for GST–1–604 with FLAG(2170–2749) in the presence of 1 mM 2-mercaptoethanol. During the incubation reaction, 20 μM IP3 or adenophostin A was added. The experimental procedure was the same as that described in (A). IP3 and adenophostin A significantly inhibited the interaction to 45±5 and 32±8% of the control respectively. Results are the means±S.D. for at least three independent experiments.