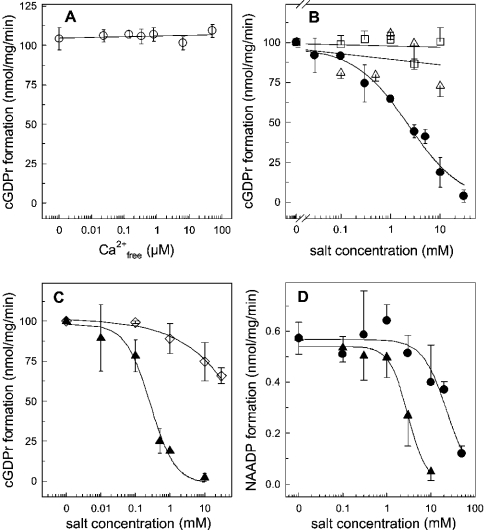
Figure 9. ADP-ribosyl cyclase activity in the presence of various ions.
HSR membranes (100 μg) were incubated in buffer A (pH 7.35) and the cyclization reaction was initiated by 100 μM NGD. (A) Enzymic formation of cGDPr was determined at various free Ca2+ concentrations, which were adjusted by the ratio of EGTA/CaCl2, according to the algorithm provided by Schoenmakers and co-workers [36]. (B) Formation of cGDPr in buffer A (pH 7.35) supplemented with ZnCl2 (•), MgCl2 (▵) or MnCl2 (□). (C) Similar to (B), the formation of cGDPr was measured in the presence of CuSO4 (▴) or potassium phosphate adjusted to pH 7.35 (□). Two experiments were pooled and cGDPr formation under control conditions was set 100% (102.8 nmol of cGDPr·mg−1·min−1). (D) The base-exchange reaction was determined in the presence of 0.5 mM NADP, 10 mM nicotinic acid, 100 μg of HSR membranes in buffer A adjusted to pH 4.3. The synthesis of NAADP was inhibited by increasing concentrations of CuSO4 (▴) and ZnCl2 (•). Results are means±S.D. for duplicates of representative experiments which were repeated twice.