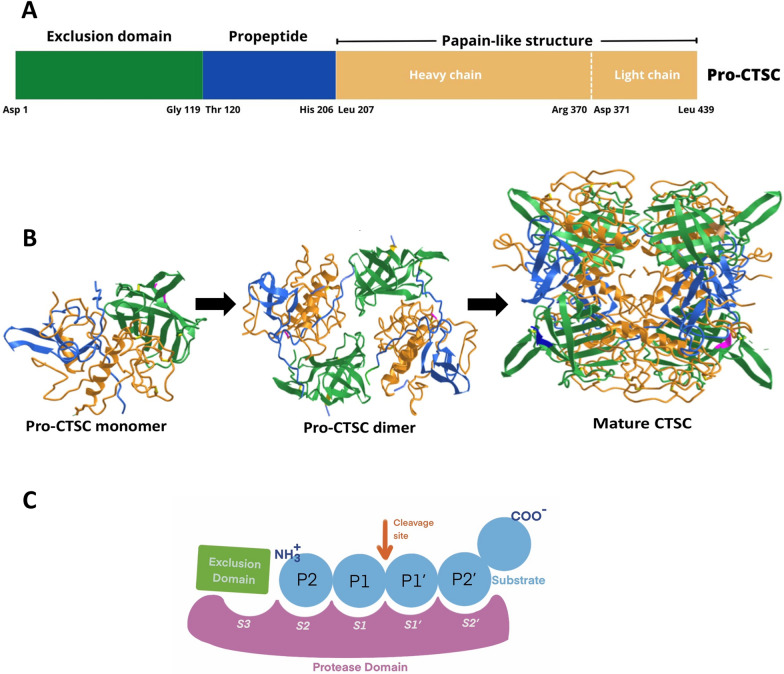
Fig. 2.
The structure of Pro-CTSC and the Process, maturation, and crystal structure of cathepsin CTSC. A The structure of Pro-CTSC is defined by distinct domains including the exclusion domain, the propeptide, and the papain-like structure region, encompassing both the heavy chain and the light chain. The exclusion domain is involved in modulating substrate specificity and stability. The propeptide is an inhibitory segment regulating enzyme activity, typically removed during maturation and activation. In the papain-like structure region, the heavy chain holds catalytic residues for proteolysis, resembling papain proteases and the light chain aids substrate binding and stability. B The maturation and structural details of cathepsin C (CTSC) involve a series of steps. Initially, the pro-CTSC monomer (modified from Protein Data Bank (PDB) entry ID: 6IC7) undergoes processing to form the pro-CTSC dimer (PDB ID: 4OEL). This dimerization process involves the convergence of two individual pro-CTSC monomers, resulting in the creation of a functional dimeric architecture, an arrangement that ensures the proper functionality of the enzyme. The pro-CTSC dimer is further processed to yield the mature CTSC enzyme (PDB ID: 61C7) with the ability to effectively participate in the degradation of proteins. C Schematic representation of the active site of papain-like peptidases modified for CTSC. The enzyme exhibited substrate binding sites labeled from S3 to S2', while substrate residues denoted by blue circles marked from P2 to P2'. An arrow indicates the cleavage site