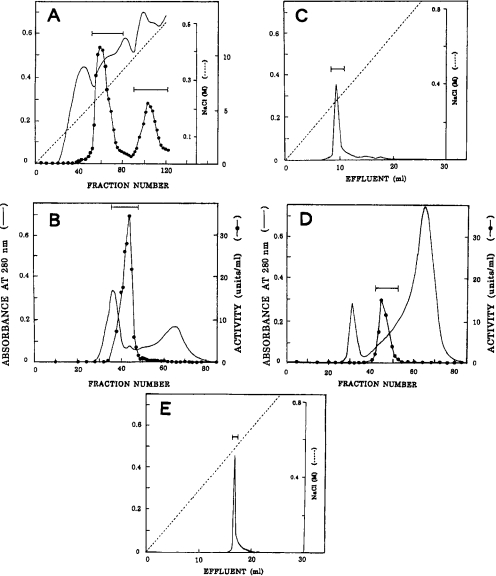
Figure 1. Purification of Nepenthes acid proteinases.
(A) DEAE-cellulose chromatography. The enzyme fraction eluted from the first DEAE-cellulose column was rechromatographed; fraction size, 10 ml. (B) Sephacryl S-200 chromatography of the major enzyme from the second DEAE-cellulose column; fraction size, 11 ml. (C) Mono Q chromatography of the major enzyme. The enzyme fraction obtained by successive chromatography on Sephacryl S-200 and pepstain–Sepharose was chromatographed. The Figure shows the result obtained with one-third of the enzyme sample. (D) Sephacryl S-200 chromatography of the minor enzyme. (E) Mono Q chromatography of the minor enzyme. The conditions were the same as in (C) except that the whole sample was used. In each chromatography, the fractions under the bar were pooled. The purified major and minor enzymes were designated nepenthesins I and II respectively.