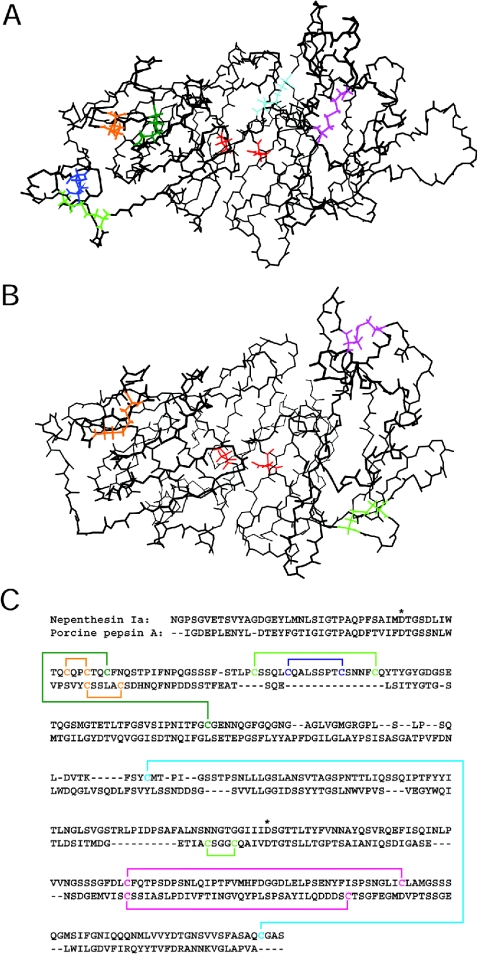
Figure 11. The backbone structure and disulphide bond pairing of nepenthesin Ia predicted by molecular modelling and comparison with porcine pepsin A.
The side chains of the cysteine residues and the putative catalytic aspartic acid residues are presented in (A, B). (A) The backbone structure of N. gracilis nepenthesin Ia. All the cysteine residues are assumed to form disulphide bonds. Cys45–Cys48 is coloured in orange, Cys51–Cys125 in green, Cys72–Cys90 in light green, Cys77–Cys85 in blue, Cys162–Cys356 in cyan and Cys276–Cys317 in magenta. The catalytic residues, Asp35 (left) and Asp237 (right), are coloured in red. (The numbering used is for the mature nepenthesin Ia.) (B) The backbone structure of porcine pepsin A. Cys45–Cys50, Cys206–Cys210 and Cys249–Cys282 (pepsin numbering) are shown in orange, light green and magenta respectively and the catalytic residues, Asp32 (left) and Asp215 (right), are shown in red. (C) Predicted arrangements of the disulphide bonds in nepenthesin Ia (upper row) and porcine pepsin A (lower row). The cysteine residues and the disulphide bonds are shown in the same colours as in (A, B). The catalytic aspartic acid residues are shown by asterisks.