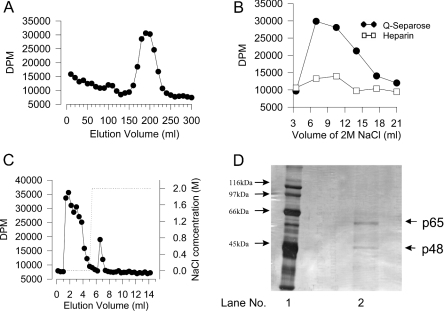
Figure 2. Purification of PLB activity.
(A) Superdex-200 fractionation. Cytosol from 5×109 cells (Dictyostelium) was precipitated with 20–45% saturated ammonium sulphate. The pellet was resuspended, dialysed, and concentrated to 10 ml [20 mM Hepes (pH 7.0)/0.02% sodium azide]. Two identical separations of the concentrate (5 ml per run) were performed using a Pharmacia Superdex-200 column at a flow rate of 1.5 ml/min. Fractions of 10 ml were collected. Assays for activity were performed on 20 μl of each fraction using didecanoyl-PC substrate. Incubations were performed for 60 min at 37 °C in the presence of 10 μM myrARF1 and 30 μM GTPγS. Water soluble metabolites were separated into choline and GPC. Only the results for GPC are shown. The results are expressed as d.p.m. released from the added 80000 d.p.m. The results shown are from one of the two identical separations. (B) heparin and Mono-Q fractionation of active Superdex-200 fractions. Peak fractions from the gel-filtration separation shown in (A) were combined. The combined material (approx. 120 ml) was applied to a 5 ml Hi-Trap heparin column connected in-line to a 5 ml Hi-Trap Q-Sepharose column at 1 ml/min. The columns were separated, and each column was washed separately with 30 ml of buffer. Proteins were eluted with 2 M NaCl (3.5 ml fractions). Fractions were desalted and the activity of 20 μl of each fraction was determined in the presence of myrARF1. The results are shown for proteins eluted from heparin and Q-Sepharose. There was negligible activity in the flow through and wash step (results not shown). (C) myrARF1 affinity purification. Peak fractions from the Q-Sepharose column shown in (B) were further fractionated using a pre-prepared myrARF1-bound NHS-affinity column. A sample of this partially purified activity (0.5 ml) was applied to the 1 ml affinity column at 1 ml/min. The column was washed with 5 ml of buffer, and then the bound protein eluted with 2 M NaCl. Fractions (200 μl) were collected for the load, wash and elution stages. Activity in every second fraction was determined. Fractions (20 μl) were assayed using didecanoyl-PC substrate in the presence of myrARF1. The results are expressed as d.p.m. released from the added 80000 d.p.m. (D) Silver-stained SDS/PAGE of purified enzyme after myrARF1 affinity purification (lane 2). Lane 1, molecular mass markers.