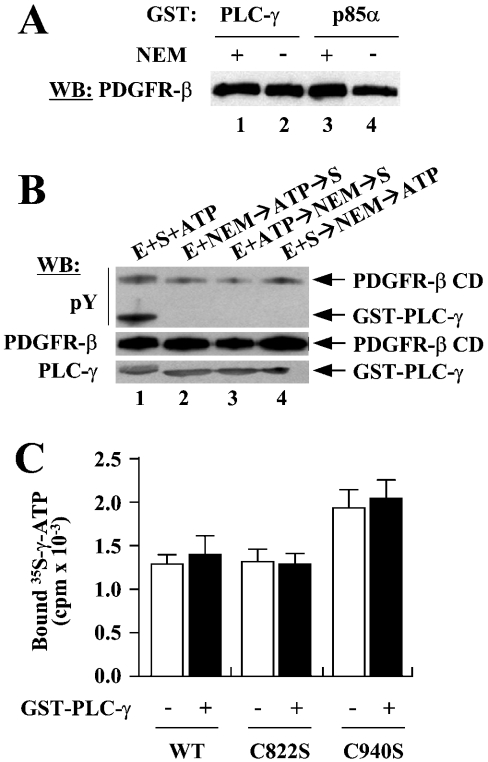
Figure 7. Neither Cys-822 nor Cys-940 is involved in substrate or ATP binding.
(A) GST pull-down assays were performed to see whether NEM blocks substrate binding. WT PDGFR-β CDs that had been incubated with (lanes 1 and 3) or without (lanes 2 and 4) NEM were subjected to pull-down assays using GST–PLC-γ or GST–p85α as bait. (B) To determine whether NEM blocks ATP binding, the effects of preoccupation of ATP on the NEM inhibition of PDGFR-β were examined. Lane 1, control reaction with no NEM treatment. Lane 2, normal reaction for the NEM inhibition of PDGFR-β; the enzyme was modified by NEM before the addition of ATP and substrate. Lane 3, PDGFR-β CD was incubated with ATP before NEM treatment and then the substrate was added to the reaction. Lane 4, PDGFR-β CD was incubated with substrate first, treated with NEM, and then ATP was added. (C) Filter-binding assays were performed to examine the effects of Cys to Ser mutations for Cys-822 or Cys-940 on ATP binding. WT, C822S or C940S were incubated with [γ-35S]ATP either with or without GST–PLC-γ, and the reactions were filtered through a nitrocellulose membrane. After intensive washing, the radioactivity retained with proteins was measured. A representative of three independent experiments is shown.